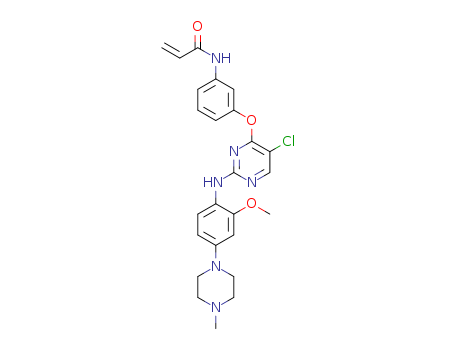

CasNo: 1213269-23-8
MF: C25H27ClN6O3
|
Biological Activity |
WZ4002 is a novel, mutant selective EGFR inhibitor. The IC50 is 2 nM/8 nM when it acts on IC50 of 2 nM/8 nM in EGFR (L858R)/(T790M), and it has no inhibitory effect on ERBB2 phosphorylation (T798I). |
|
In vitro studies |
WZ4002 also inhibit other EGFR genotypes. For instance, the IC50 is 2nM and 6nM respectively when it acts on E746_A750 and E746_A750/T790M. Besides, the IC50 is 32nM when it acts on wild-type EGFR. It could inhibit the phosphorylation of EGFR, AKT and ERK1/2 when it acts on non-small cell lung cancer (NSCLC). WZ4002 also could inhibit the phosphorylation of EGFR when it acts on NIH-3T3 cells expressing different EGFRT790M mutant alleles. The dissociation constant of kinase was 95% higher when in the test when WZ4002 involved than that in the test DMSO involved instead. Because in aniline substitution in the C2 WZ4002 could obtain a methoxy group when it acts on EGFR, thus it’s more effective when it acts on EGFR than WZ3146. Compared with the quinazoline inhibitors, the efficiency of WZ4002 is 100 times lower than when it acts on wild-type EGFR. The inhibitory effect of WZ4002 on EGFR kinase activity of recombinant L858R/T790M protein is much higher than restrainable wild-type EGFR’s. However HKI-272 and Gefitinib are happened to have opposite inhibitory effect compared with WZ4002. In addition, phosphorylation of EGFR in H1975 and HCC827 cells of anti-Src TKI could be completely inhibited by third-generation EGFR TKI and WZ4002. |
|
In vivo studies |
When treated with T790M mutant mouse model for 2 weeks and had been used to study the efficacy of WZ4002, it is found that treatment with WZ4002 resulted in more effective significant tumor regression compared to the control group. When treated with low dose WZ4002 and high dose WZ4002, it would resulted in a 26% and 36% decrease in average uptake respectively. |
|
references |
[1]lee hj, schaefer g, heffron tp, shao l, ye x, sideris s, malek s, chan e, merchant m, la h, ubhayakar s, yauch rl, pirazzoli v, politi k, settleman j. noncovalent wild-type-sparing inhibitors of egfr t790m. cancer discov. 2013 feb;3(2):168-81. doi: 10.1158/2159-8290.cd-12-0357. epub 2012 dec 10.[2]zhou w, ercan d, chen l, yun ch, li d, capelletti m, cortot ab, chirieac l, iacob re, padera r, engen jr, wong kk, eck mj, gray ns, j?nne pa. novel mutant-selective egfr kinase inhibitors against egfr t790m. nature. 2009 dec 24;462(7276):1070-4. doi: 10.1038/nature08622.[3]zannetti a, iommelli f, speranza a, salvatore m, del vecchio s. 3'-deoxy-3'-18f-fluorothymidine pet/ct to guide therapy with epidermal growth factor receptor antagonists and bcl-xl inhibitors in non-small cell lung cancer. j nucl med. 2012 mar;53(3):443-50. doi: 10.2967/jnumed.111.096503. epub 2012 feb 13. |
InChI:InChI=1S/C25H27ClN6O3/c1-4-23(33)28-17-6-5-7-19(14-17)35-24-20(26)16-27-25(30-24)29-21-9-8-18(15-22(21)34-3)32-12-10-31(2)11-13-32/h4-9,14-16H,1,10-13H2,2-3H3,(H,28,33)(H,27,29,30)
A series of thirty two anilinopyrimidine...
Novel compounds 12a-i were synthesized a...
Targeting the epidermal growth factor re...
The present invention relates to novel p...
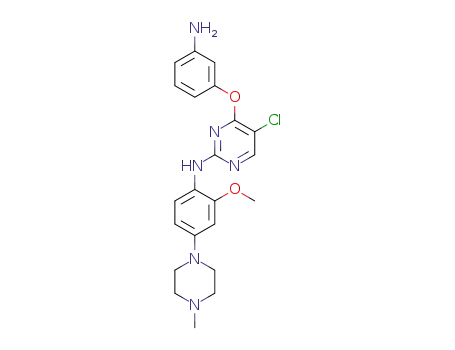
4-(3-aminophenoxy)-5-chloro-N-(2-methoxy-4-(4-methylpiperazin-1-yl)phenyl) pyrimidin-2-amine

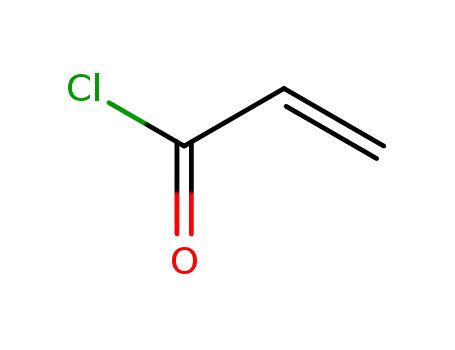
acryloyl chloride

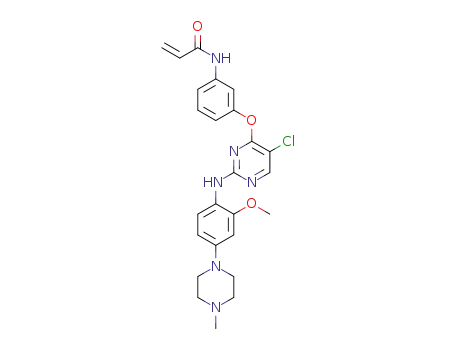
N-(3-(5-chloro-2-(2-methoxy-4-(4-methylpiperazin-1-yl)phenylamino)pyrimidin-4-yloxy)phenyl)acrylamide
| Conditions | Yield |
|---|---|
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 0 ℃;
for 2h;
|
36% |
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 0 ℃;
for 1h;
|
|
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
|
|
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 1h;
|
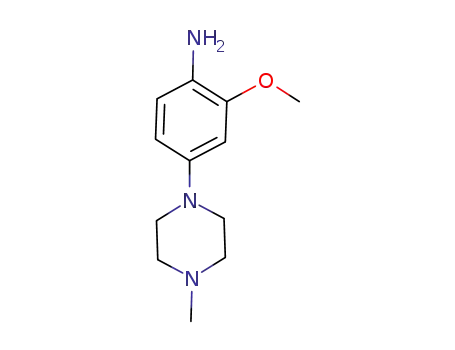
2-methoxy-4-(4-methylpiperazin-1-yl)aniline


N-(3-(5-chloro-2-(2-methoxy-4-(4-methylpiperazin-1-yl)phenylamino)pyrimidin-4-yloxy)phenyl)acrylamide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: trifluoroacetic acid / iso-butanol / 100 °C
2: platinum(IV) oxide; hydrogen / methanol
3: N-ethyl-N,N-diisopropylamine / dichloromethane
With
platinum(IV) oxide; hydrogen; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid;
In
methanol; dichloromethane; iso-butanol;
|
|
|
Multi-step reaction with 3 steps
1: trifluoroacetic acid / iso-butanol / 3 h / Reflux
2: iron; ammonium chloride / tetrahydrofuran; water / 3 h / Reflux
3: triethylamine / dichloromethane / 1 h / 0 °C
With
iron; ammonium chloride; triethylamine; trifluoroacetic acid;
In
tetrahydrofuran; dichloromethane; water; iso-butanol;
|
|
|
Multi-step reaction with 3 steps
1: trifluoroacetic acid / iso-butanol / 4 h / 100 °C
2: iron; ammonium chloride / tetrahydrofuran; water / 4 h / 65 °C
3: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C
With
iron; ammonium chloride; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid;
In
tetrahydrofuran; dichloromethane; water; iso-butanol;
|

4-(3-aminophenoxy)-5-chloro-N-(2-methoxy-4-(4-methylpiperazin-1-yl)phenyl) pyrimidin-2-amine

acryloyl chloride

2-methoxy-4-(4-methylpiperazin-1-yl)aniline
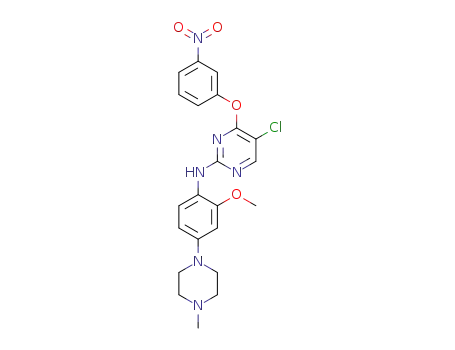
5-chloro-N-(2-methoxy-4-(4-methylpiperazin-1-yl)phenyl)-4-(3-nitrophenoxy)pyrimidin-2-amine
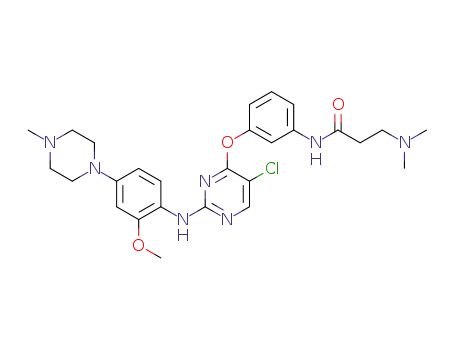
N-(3-((5-chloro-2-((2-methoxy-4-(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)oxy)phenyl)-3-(dimethylamino)propanamide
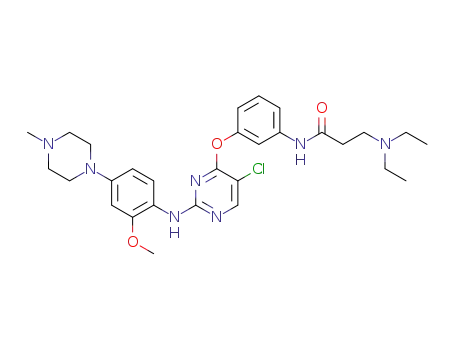
N-(3-((5-chloro-2-((2-methoxy-4-(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)oxy)phenyl)-3-(diethylamino)propanamide
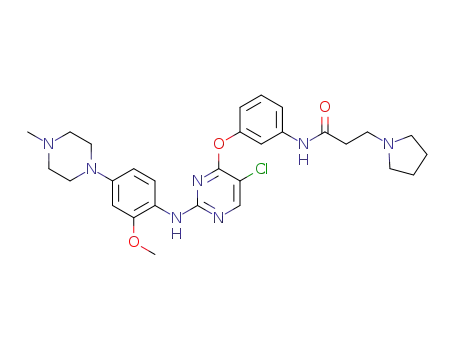
N-(3-((5-chloro-2-((2-methoxy-4-(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)oxy)phenyl)-3-(pyrrolidin-1-yl)propanamide
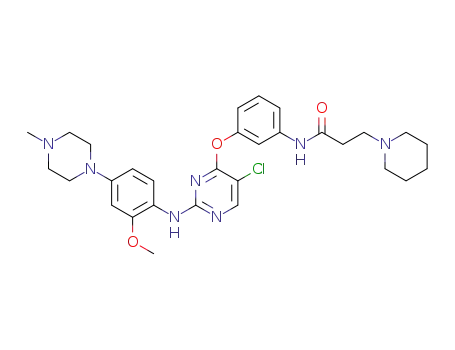
N-(3-((5-chloro-2-((2-methoxy-4-(4-methylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)oxy)phenyl)-3-(piperidin-1-yl)propanamide