Your Location:Home > Products > Pharmaceutical Intermediates
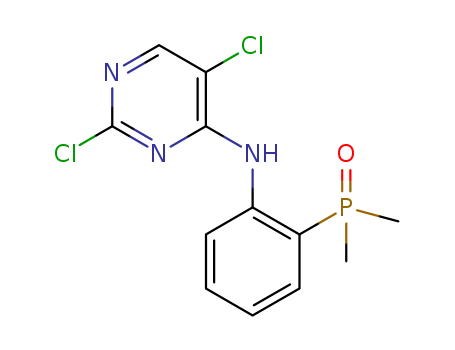

CasNo: 1197953-49-3
Disclosed is an application of a series ...
A series of brigatinib derivatives were ...
The invention relates to the technical f...
Provided are compounds of Formula I, met...
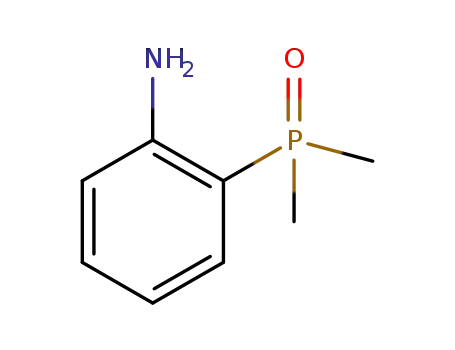
(2-aminophenyl)dimethyl phosphorus oxide

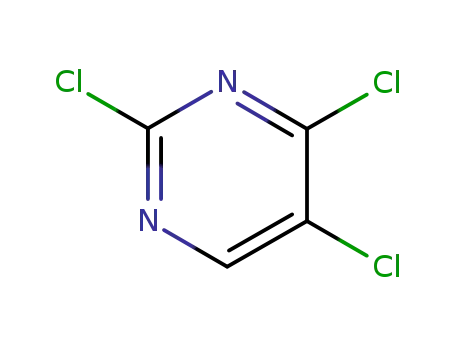
2,4,5-trichloropyrimidine

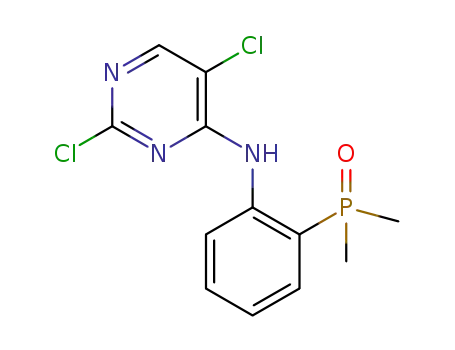
(2-((2,5-dichloropyrimidin-4-yl)amino)phenyl)dimethyl phosphine oxide
| Conditions | Yield |
|---|---|
|
With
dipotassium hydrogenphosphate;
In
N,N-dimethyl-formamide;
at 65 ℃;
for 4h;
|
90.3% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 4h;
|
84.6% |
|
With
tetra(n-butyl)ammonium hydrogensulfate; potassium carbonate;
In
N,N-dimethyl-formamide;
at 65 ℃;
|
84% |
|
With
tetra(n-butyl)ammonium hydrogensulfate; potassium carbonate;
In
N,N-dimethyl-formamide;
at 65 ℃;
for 8h;
|
78% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 5h;
|
69% |
|
With
N-ethyl-N,N-diisopropylamine;
In
N,N-dimethyl-formamide;
at 16 - 70 ℃;
for 16h;
|
68.4% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 5h;
|
61% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 5h;
|
61% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 5h;
|
61% |
|
With
N-ethyl-N,N-diisopropylamine;
for 12h;
Reflux;
|
50% |
|
With
tetra(n-butyl)ammonium hydrogensulfate; potassium carbonate;
In
N,N-dimethyl-formamide;
at 65 ℃;
Reagent/catalyst;
Concentration;
Temperature;
Solvent;
|
|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 70 ℃;
|
|
|
at 75 ℃;
for 6h;
Concentration;
|
17 g |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 60 ℃;
|
0.7 g |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 8h;
|
3 g |
|
(2-aminophenyl)dimethyl phosphorus oxide;
With
sodium hydride;
In
N,N-dimethyl-formamide;
at 0 ℃;
for 0.5h;
2,4,5-trichloropyrimidine;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
1.6 g |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 8h;
|
3 g |
|
(2-aminophenyl)dimethyl phosphorus oxide;
With
sodium hydride;
In
N,N-dimethyl-formamide;
at 0 ℃;
for 0.5h;
2,4,5-trichloropyrimidine;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
1.6 g |
|
With
N-ethyl-N,N-diisopropylamine;
In
N,N-dimethyl-formamide;
at 16 - 70 ℃;
for 16h;
|
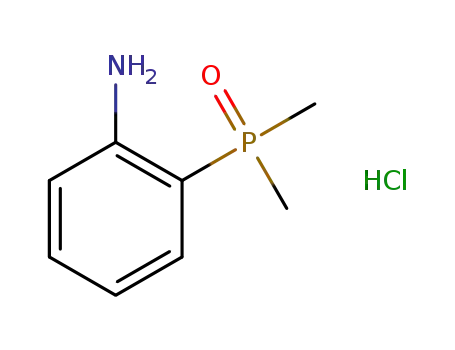
(2-aminophenyl)dimethylphosphine oxide hydrochloride


2,4,5-trichloropyrimidine


(2-((2,5-dichloropyrimidin-4-yl)amino)phenyl)dimethyl phosphine oxide
| Conditions | Yield |
|---|---|
|
With
N-ethyl-N,N-diisopropylamine;
In
ethanol;
at 80 ℃;
for 18h;
Large scale;
|
79% |
|
With
N-ethyl-N,N-diisopropylamine;
In
ethanol;
at 80 ℃;
for 18h;
Large scale;
|
79% |
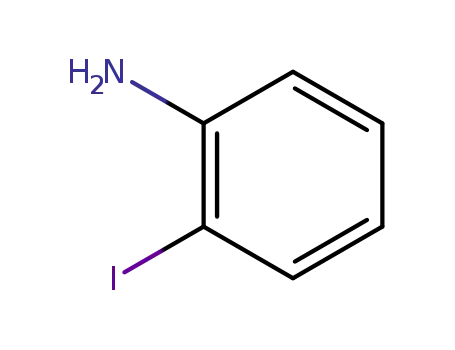
2-iodophenylamine

(2-aminophenyl)dimethyl phosphorus oxide

2,4,5-trichloropyrimidine
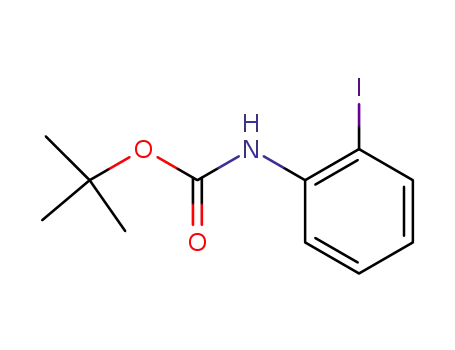
tert-butyl 2-iodophenylcarbamate
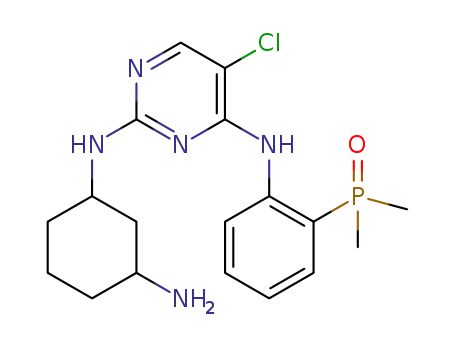
C18H25ClN5OP
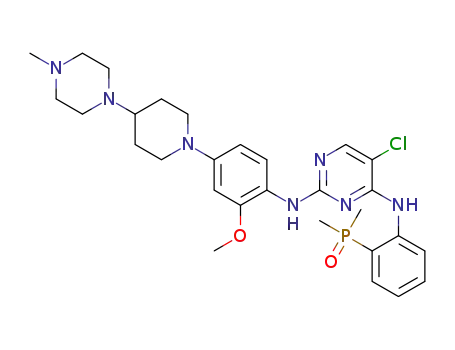
5-chloro-N4-[2-(dimethylphosphonyl)phenyl]-N2-{2-methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]phenyl}pyrimidine-2,4-diamine
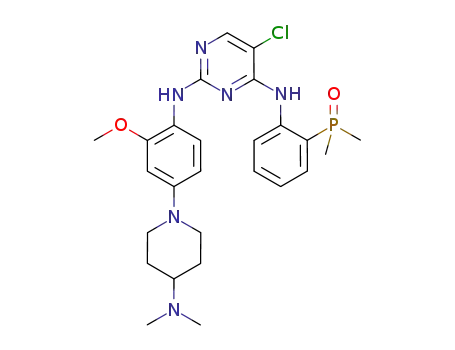
(2-((5-chloro-2-((4-(4-(dimethylamino)piperidin-1-yl)-2-methoxyphenyl )amino)pyrimidin-4-yl)amino)phenyl)dimethylphosphine oxide