Your Location:Home > Products > Pharmaceutical Intermediates
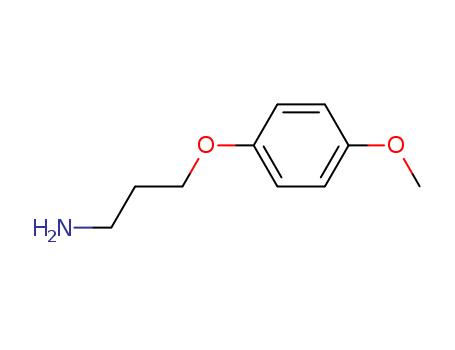

CasNo: 100841-00-7
MF: C10H15NO2
|
General Description |
3-(4-methoxyphenoxy)propan-1-amine, also known as 2-(4-methoxyphenoxy)propan-1-amine or PPG-14 butyl ether, is a chemical compound with the molecular formula C10H15NO2. It belongs to the class of amines and is derived from propan-1-amine. It is commonly used as an ingredient in various personal care and cosmetic products, suchagent for liquid household and personal care products, as well as an ingredient in lubricants and paints. It is also sometimes used as a fragrance ingredient in perfumes and colognes. |
InChI:InChI=1/C10H15NO2/c1-12-9-3-5-10(6-4-9)13-8-2-7-11/h3-6H,2,7-8,11H2,1H3
An important determinant of the growth i...
Enantioselective synthesis of enantiomer...
A process for obtaining a compound (1) a...
The present invention provides a product...
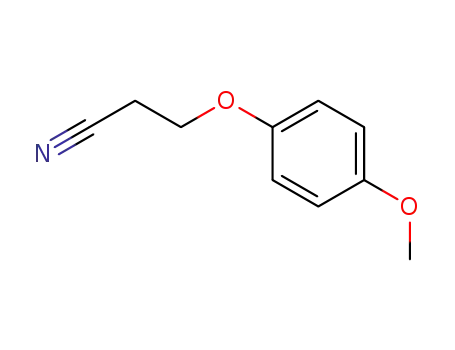
2-(4-Methoxyphenoxy)cyanoethane

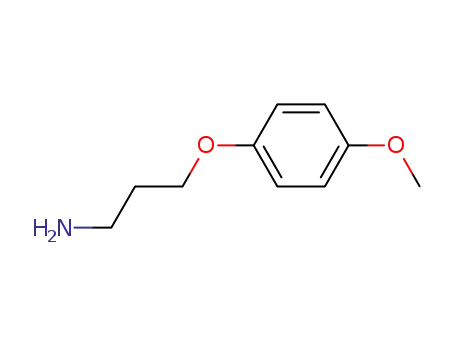
3-(4-methoxyphenoxy)propan-1-amine
| Conditions | Yield |
|---|---|
|
With
borane-THF;
In
tetrahydrofuran;
at 65 ℃;
for 5h;
|
90% |
|
2-(4-Methoxyphenoxy)cyanoethane;
With
borane-THF;
In
tetrahydrofuran;
at 80 ℃;
for 3.16667h;
With
sodium hydroxide; water;
In
tetrahydrofuran;
at 0 - 80 ℃;
for 12.4h;
|
79% |
|
With
sodium hydroxide;
In
tetrahydrofuran; toluene;
|
79% |
|
2-(4-Methoxyphenoxy)cyanoethane;
With
borane-THF;
In
tetrahydrofuran;
at 80 ℃;
for 3.16h;
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 0 - 80 ℃;
for 12.25h;
|
79% |
|
With
borane-THF;
In
tetrahydrofuran;
|
![<i>N</i>-[3-(4-methoxy-phenoxy)-propyl]-phthalimide](/upload/2025/5/4bb6f7e6-bdee-4e06-822f-dbe6233c7791.png)
N-[3-(4-methoxy-phenoxy)-propyl]-phthalimide


3-(4-methoxyphenoxy)propan-1-amine
| Conditions | Yield |
|---|---|
|
With
methylamine;
In
methanol;
at 20 ℃;
for 4h;
|
58% |
|
With
hydrazine hydrate;
In
ethanol;
Heating;
|
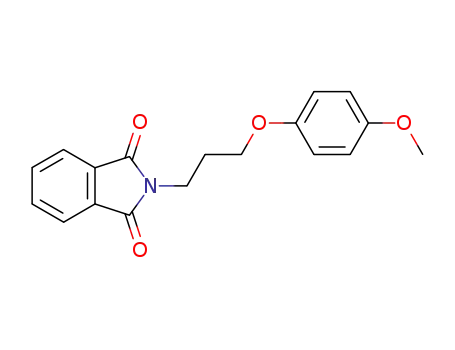
N-[3-(4-methoxy-phenoxy)-propyl]-phthalimide

2-(4-Methoxyphenoxy)cyanoethane
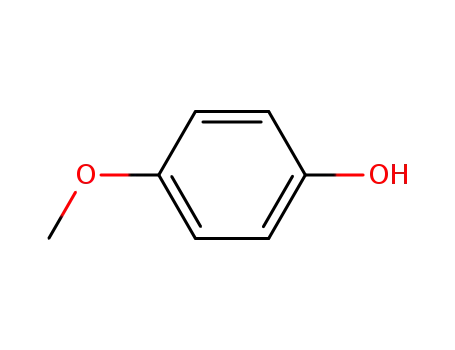
4-methoxy-phenol
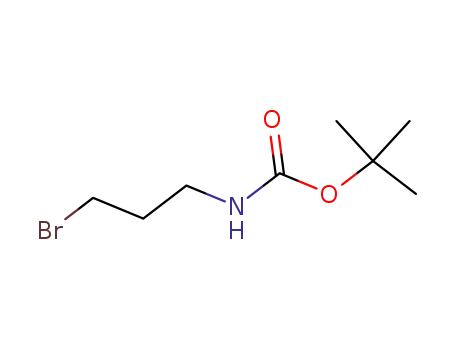
3-bromopropylamine tert-butylcarbamate
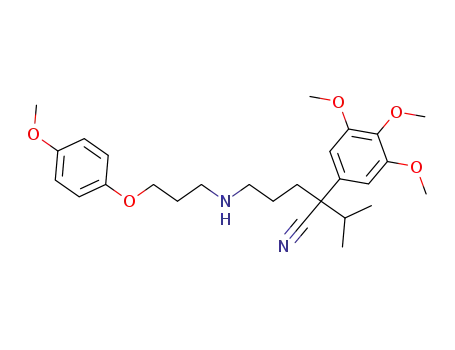
2-Isopropyl-5-[3-(4-methoxy-phenoxy)-propylamino]-2-(3,4,5-trimethoxy-phenyl)-pentanenitrile
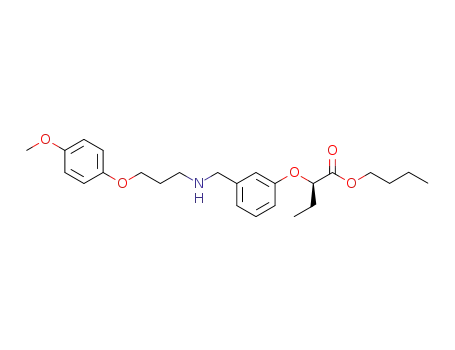
n-butyl (R)-2-[3-[N-[3-(4-methoxyphenoxy)propyl]aminomethyl]phenoxy]butyrate
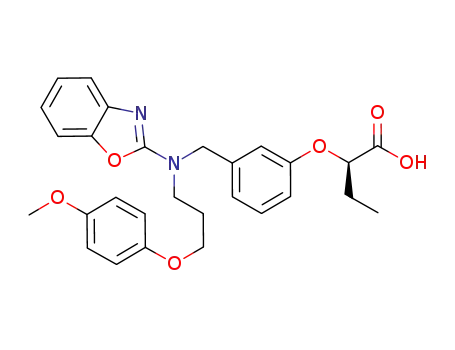
(2R)-2-[3-[[1,3-benzoxazol-2-yl-[3-(4-methoxyphenoxy)propyl]amino]methyl]phenoxy]butanoic acid
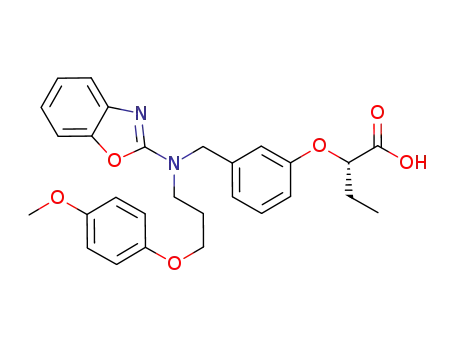
C28H30N2O6