CasNo: 1197953-54-0
MF: C29H39ClN7O2P
The invention relates to the technical f...
The invention relates to the technical f...
In the treatment of echinoderm microtubu...
Crystalline forms of brigatinib, pharmac...
![2-methoxy-4-[4-(4-methylpiperazin-1-yl)-piperidin-1-yl]-phenylamine](/upload/2025/5/c25951f3-9577-4d6c-b8e9-a0a93fca8a8a.png)
2-methoxy-4-[4-(4-methylpiperazin-1-yl)-piperidin-1-yl]-phenylamine

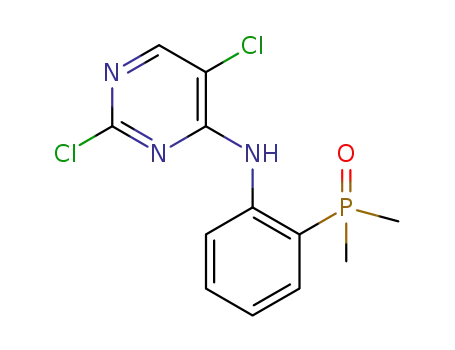
(2-((2,5-dichloropyrimidin-4-yl)amino)phenyl)dimethyl phosphine oxide

5-chloro-N4-[2-(dimethylphosphonyl)phenyl]-N2-{2-methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]phenyl}pyrimidine-2,4-diamine
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
1,2-dimethoxyethane; ethanol;
at 120 ℃;
for 5h;
Sealed tube;
|
80.3% |
|
With
hydrogenchloride;
In
ethanol; 2-methoxy-ethanol;
at 120 ℃;
for 5.5h;
Sealed tube;
|
66% |
|
With
hydrogenchloride;
In
ethanol; 2-methoxy-ethanol;
at 120 ℃;
for 6h;
Sealed tube;
|
65% |
|
With
hydrogenchloride;
In
ethanol; 2-methoxy-ethanol;
at 120 ℃;
|
65% |
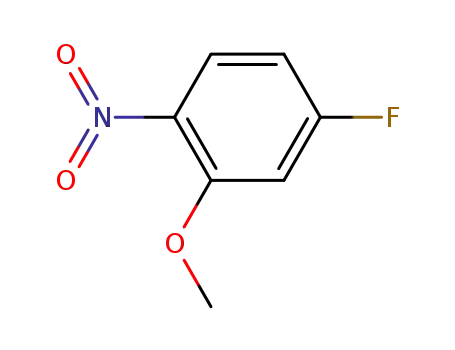
4-fluoro-2-methoxy-1-nitrobenzene

5-chloro-N4-[2-(dimethylphosphonyl)phenyl]-N2-{2-methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]phenyl}pyrimidine-2,4-diamine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: potassium carbonate / N,N-dimethyl-formamide / 18 h / 120 °C
2: hydrogen; palladium on activated charcoal / ethanol / 3 h / 2585.81 Torr
3: hydrogenchloride / ethanol; 2-methoxy-ethanol / 5.5 h / 120 °C / Sealed tube
With
hydrogenchloride; palladium on activated charcoal; hydrogen; potassium carbonate;
In
ethanol; 2-methoxy-ethanol; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate / acetonitrile / 13 h / 80 °C
2: hydrogen; palladium 10% on activated carbon / ethanol / 2.5 h
3: hydrogenchloride / ethanol; 2-methoxy-ethanol / 6 h / 120 °C / Sealed tube
With
hydrogenchloride; palladium 10% on activated carbon; hydrogen; potassium carbonate;
In
ethanol; 2-methoxy-ethanol; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate / acetonitrile / Reflux
2: palladium 10% on activated carbon; hydrogen / ethanol; water / 10.34 Torr
3: hydrogenchloride / ethanol; 2-methoxy-ethanol / 120 °C
With
hydrogenchloride; palladium 10% on activated carbon; hydrogen; potassium carbonate;
In
ethanol; 2-methoxy-ethanol; water; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate / acetonitrile / 4.5 h / Reflux
2: hydrogen; 15% palladium on carbon / ethanol / 2.5 h / 25 °C / 517.16 Torr
3: triethylamine / ethanol; 1,2-dimethoxyethane / 5 h / 120 °C / Sealed tube
With
15% palladium on carbon; hydrogen; potassium carbonate; triethylamine;
In
1,2-dimethoxyethane; ethanol; acetonitrile;
|
|
|
Multi-step reaction with 5 steps
1.1: potassium carbonate / dimethyl sulfoxide / 5 h / 60 °C
2.1: palladium on activated charcoal; hydrogen / tetrahydrofuran / 3.5 h / 20 °C
2.2: 1.5 h / 35 °C
3.1: trifluoroacetic acid / ethanol / 16 h / Inert atmosphere; Reflux
4.1: Jones reagent / acetone / 0.5 h / 20 - 25 °C
5.1: 1,4-dioxane / 0.33 h
5.2: 20 °C
With
Jones reagent; palladium on activated charcoal; hydrogen; potassium carbonate; trifluoroacetic acid;
In
tetrahydrofuran; 1,4-dioxane; ethanol; dimethyl sulfoxide; acetone;
|

(2-((2,5-dichloropyrimidin-4-yl)amino)phenyl)dimethyl phosphine oxide
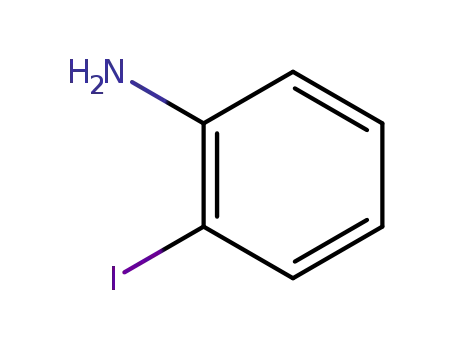
2-iodophenylamine
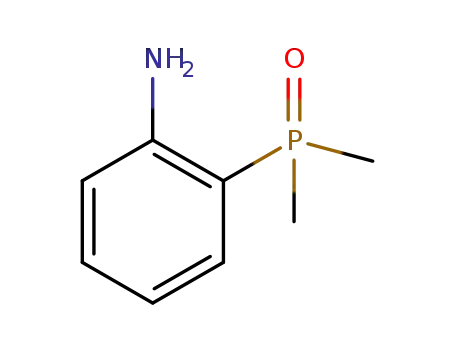
(2-aminophenyl)dimethyl phosphorus oxide

4-fluoro-2-methoxy-1-nitrobenzene