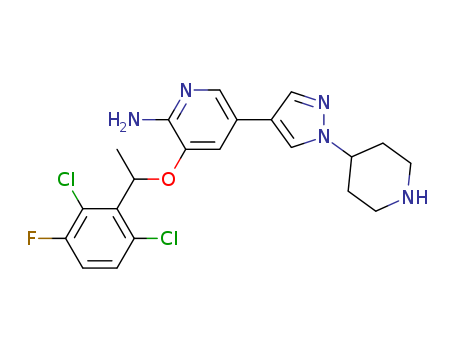

CasNo: 877399-52-5
MF: C21H22Cl2FN5O
Quality products make an important contribution to long-term revenue and profitability. Manufacturer supply Crizotinib 877399-52-5 with sufficient stock and high standard
Crizotinib (Xalkori(R), Pfizer), approved in 2011, was the first approved inhibitor targeting anaplastic lymphoma kinase (ALK). ROS protooncogene 1-encoded kinase (ROS1) of the tyrosine kinase insulin receptor class and MET proto-oncogene-encoded kinase of the hepatocyte growth factor receptor (HGFR) class are other kinases targeted by crizotinib.When approved in 2011, crizotinib was the first drug specifically targeting NSCLC patients. However, resistance to crizotinib was usually observed in approximately 8 months after initial application and more than half of crizotinib-treated patients experienced gastrointestinal side effects. In 2016,crizotinib was additionally approved for ROS1-positive NSCLC by FDA.
Crizotinib is a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). Crizotinib is a potential antitumor agent. In August 2011, the United States FDA approved crizotinib for the treatment of anaplastic lymphoma kinase (ALK) rearranged non-small-cell lung cancer (NSCLC).
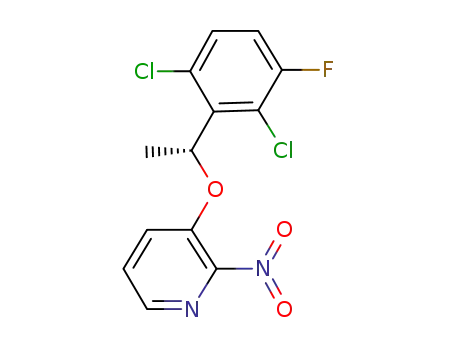
(R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethyoxyl)-2-nitropyridine

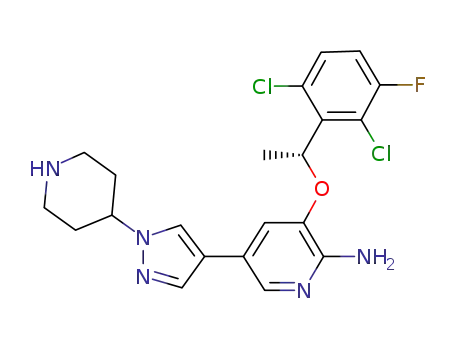
crizotinib
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1: iron; acetic acid / ethanol / Inert atmosphere; Reflux
2: N-Bromosuccinimide / acetonitrile / 0 °C / Inert atmosphere
3: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; caesium carbonate / 1,2-dimethoxyethane; water / 3 h / 90 °C / Inert atmosphere
4: hydrogenchloride / 1,4-dioxane; dichloromethane; water / 4 h / 0 - 20 °C / Inert atmosphere
With
hydrogenchloride; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; N-Bromosuccinimide; iron; caesium carbonate; acetic acid;
In
1,4-dioxane; 1,2-dimethoxyethane; ethanol; dichloromethane; water; acetonitrile;
3: Suzuki coupling;
|
|
|
Multi-step reaction with 4 steps
1.1: hydrogen / methanol / 25 - 50 °C / Large scale reaction
2.1: N-Bromosuccinimide / dichloromethane; acetonitrile / 1.42 h / -15 - -10 °C / Large scale reaction
2.2: 0.17 h / 0 °C / Large scale reaction
2.3: 0.25 h / Large scale reaction
3.1: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; tetrabutylammomium bromide; caesium carbonate / water; toluene / 4.5 h / 22 - 70 °C / Inert atmosphere; Large scale reaction
4.1: ethanol; acetyl chloride / dichloromethane; ethyl acetate / 5 h / 0 - 25 °C / Large scale reaction
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; N-Bromosuccinimide; ethanol; tetrabutylammomium bromide; hydrogen; caesium carbonate; acetyl chloride;
In
methanol; dichloromethane; water; ethyl acetate; toluene; acetonitrile;
3.1: Suzuki-Miyaura coupling;
|
|
|
Multi-step reaction with 5 steps
1.1: hydrogen / ethanol / 50 °C / 11251.1 Torr / Autoclave
2.1: N-Bromosuccinimide / acetonitrile / 0.5 h / -15 - 10 °C
3.1: caesium carbonate / 5,5-dimethyl-1,3-cyclohexadiene / 1 h / 20 °C / Inert atmosphere
3.2: 3 h / Inert atmosphere; Reflux
4.1: ethanol / 18 h / Inert atmosphere; Reflux
5.1: 5,5-dimethyl-1,3-cyclohexadiene / 16 h / Inert atmosphere; Reflux
With
N-Bromosuccinimide; hydrogen; caesium carbonate;
In
5,5-dimethyl-1,3-cyclohexadiene; ethanol; acetonitrile;
|
|
|
Multi-step reaction with 4 steps
1: acetic acid; iron / ethanol / 0.5 h / 40 - 60 °C
2: N-Bromosuccinimide / tetrachloromethane / 0 °C
3: sodium carbonate; bis-triphenylphosphine-palladium(II) chloride / water; N,N-dimethyl-formamide / 16 h / Inert atmosphere
4: hydrogenchloride / ethyl acetate / 0.33 h / 0 °C
With
hydrogenchloride; bis-triphenylphosphine-palladium(II) chloride; N-Bromosuccinimide; iron; sodium carbonate; acetic acid;
In
tetrachloromethane; ethanol; water; ethyl acetate; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 4 steps
1: acetic acid; iron / ethanol / 5 h / 20 °C / Large scale
2: N-Bromosuccinimide / dichloromethane; acetonitrile / 1 h / -15 - -10 °C / Large scale
3: caesium carbonate; tetrabutylammomium bromide; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / benzene; water / 5 h / 70 °C / Inert atmosphere
4: hydrogenchloride / ethanol; dichloromethane; water / 3 h / 0 - 25 °C
With
hydrogenchloride; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; N-Bromosuccinimide; tetrabutylammomium bromide; iron; caesium carbonate; acetic acid;
In
ethanol; dichloromethane; water; acetonitrile; benzene;
|
|
|
Multi-step reaction with 4 steps
1.1: hydrogen / methanol; dichloromethane / 0 h / 20 - 50 °C / 5400.54 - 6225.62 Torr / Flow reactor
2.1: N-Bromosuccinimide / dichloromethane; acetonitrile / 0.5 h / 0 - 25 °C
2.2: 1 h / 25 °C
3.1: tetrabutylammomium bromide; caesium carbonate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / toluene; water / 6 h / 80 °C / Inert atmosphere
4.1: hydrogenchloride / dichloromethane; 1,4-dioxane; ethanol / -5 - 25 °C
With
hydrogenchloride; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; N-Bromosuccinimide; tetrabutylammomium bromide; hydrogen; caesium carbonate;
In
1,4-dioxane; methanol; ethanol; dichloromethane; water; toluene; acetonitrile;
|
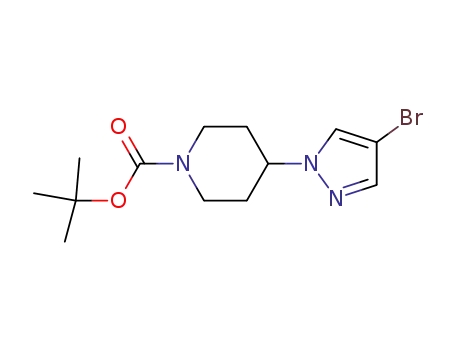
4-(4-bromo-1H-pyrazol-1-yl)piperidine-1-carboxylic acid tert-butyl ester


crizotinib
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: n-butyllithium / tetrahydrofuran; hexane / 3 h / -70 - 20 °C
2: tetrabutylammomium bromide; caesium carbonate; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 / toluene; water / 3 h / 20 - 90 °C / Inert atmosphere
3: hydrogenchloride / dichloromethane; 1,4-dioxane / 4 h / 0 - 20 °C
With
hydrogenchloride; n-butyllithium; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; tetrabutylammomium bromide; caesium carbonate;
In
tetrahydrofuran; 1,4-dioxane; hexane; dichloromethane; water; toluene;
|
|
|
Multi-step reaction with 3 steps
1.1: isopropylmagnesium chloride; lithium chloride / tetrahydrofuran / 12 h / 20 - 30 °C
1.2: 6 h / 20 - 30 °C
2.1: bis-triphenylphosphine-palladium(II) chloride; sodium carbonate / N,N-dimethyl-formamide; water / 3 h / 60 °C
3.1: hydrogenchloride / dichloromethane; ethanol / 2 h / 20 - 30 °C
With
hydrogenchloride; bis-triphenylphosphine-palladium(II) chloride; isopropylmagnesium chloride; sodium carbonate; lithium chloride;
In
tetrahydrofuran; ethanol; dichloromethane; water; N,N-dimethyl-formamide;
2.1: |Suzuki Coupling;
|
|
|
Multi-step reaction with 3 steps
1.1: isopropylmagnesium chloride; lithium chloride / tetrahydrofuran / 12 h / 20 - 30 °C
1.2: 6 h / 20 - 30 °C
2.1: bis-triphenylphosphine-palladium(II) chloride; sodium carbonate / N,N-dimethyl-formamide; water / 3 h / 60 °C
3.1: hydrogenchloride / dichloromethane; ethanol / 2 h / 20 - 30 °C
With
hydrogenchloride; bis-triphenylphosphine-palladium(II) chloride; isopropylmagnesium chloride; sodium carbonate; lithium chloride;
In
tetrahydrofuran; ethanol; dichloromethane; water; N,N-dimethyl-formamide;
2.1: |Suzuki Coupling;
|
|
|
Multi-step reaction with 3 steps
1.1: isopropylmagnesium chloride / tetrahydrofuran / 12 h / 20 °C / Autoclave; Large scale
1.2: 12 h / Large scale
1.3: Large scale
2.1: tetrabutylammomium bromide; sodium carbonate; bis-triphenylphosphine-palladium(II) chloride / water; toluene / 70 °C
3.1: trifluoroacetic acid / dichloromethane / 0 - 20 °C
With
bis-triphenylphosphine-palladium(II) chloride; tetrabutylammomium bromide; isopropylmagnesium chloride; sodium carbonate; trifluoroacetic acid;
In
tetrahydrofuran; dichloromethane; water; toluene;
2.1: |Suzuki Coupling;
|
|
|
Multi-step reaction with 3 steps
1: isopropylmagnesium chloride / tetrahydrofuran / 9 h / -10 - 30 °C / Inert atmosphere; Large scale
2: caesium carbonate; tetrabutylammomium bromide; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / benzene; water / 5 h / 70 °C / Inert atmosphere
3: hydrogenchloride / ethanol; dichloromethane; water / 3 h / 0 - 25 °C
With
hydrogenchloride; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; tetrabutylammomium bromide; isopropylmagnesium chloride; caesium carbonate;
In
tetrahydrofuran; ethanol; dichloromethane; water; benzene;
|
The CAS number of Crizotinib is 877399-52-5.
More information of Crizotinib 877399-52-5 are:
|
CAS Number |
877399-52-5 |
|
Density |
1.475 g/cm3 |
|
Melting Point |
192 °C |
|
Boiling Point |
599.177 °C at 760 mmHg |
|
Flash Point |
316.171 °C |
|
Vapor Pressure |
0mmHg at 25°C |
|
Refractive Index |
1.673 |
|
HS CODE |
29333990 |
|
PSA |
77.99000 |
|
LogP |
5.94770 |
|
Pka |
9.81±0.10(Predicted) |
Synonyms for Crizotinib 877399-52-5:PF-02341066;[3-[[(R)-1-(2,6-Dichloro-3-fluorophenyl)ethyl]oxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-yl]amine;3-[(1R)-1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-amine;2-Pyridinamine,3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-piperidinyl)-1H-pyrazol-4-yl]-;
The chemical formula of Crizotinib is C21H22Cl2FN5O which containing 21 Carbon atoms,22 Hydrogen atoms,2 Chlorine atoms,1 Fluorine atoms,5 Nitrogen atoms and 1 Oxygen atoms,and the molecular weight of Crizotinib is 450.343.
Crizotinib is a selective tyrosine kinase receptor inhibitor used in the therapy of selected cases of advanced non-small cell lung cancer. It is a dual ATP competitive inhibitor of tyrosine kinases c-MET (Mesenchymal-Epithelial Transition Factor) kinase (cellular IC50=8 nM) and ALK (cellular IC50=20 nM), both of which are important targets for cancer chemotherapy. When crizotinib was tested for selectivity versus other kinases it was found to have enzyme IC50's within 100-fold multiples of c-MET for 13 of the 120 kinases tested. In cellular assays, crizotinib was found to inhibit RON (recepteur d’origine nantais) kinase with a 10-fold selectivity window over c-MET. Altogether, this agent inhibits tumor cell growth.XALKORI?(crizotinib) is a prescription medicine used to treat people with non-small cell lung cancer (NSCLC) that has spread to other parts of the body and is caused by a defect in either a gene called ALK (anaplastic lymphoma kinase) or a gene called ROS1. It is not known if XALKORI is safe and effective in children.
InChI:InChI=1/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1
Relevant articles related to Crizotinib:
|
Article |
Source |
|
Synthesis of a Crizotinib Intermediate via Highly Efficient Catalytic Hydrogenation in Continuous Flow |
Chen, Jianli,Cheng, Pengfei,Su, Weike,Xie, Xiaoxuan,Xu, Feng,Yu, Zhiqun , p. 2252 - 2259 (2020/11/26) |
|
Design, synthesis and structure-activity relationship study of aminopyridine derivatives as novel inhibitors of Janus kinase 2 |
Wang, Wanqi,Diao, Yanyan,Li, Wenjie,Luo, Yating,Yang, Tingyuan,Zhao, Yuyu,Qi, TianTian,Xu, Fangling,Ma, Xiangyu,Ge, Huan,Liang, Yingfan,Zhao, Zhenjiang,Liang, Xin,Wang, Rui,Zhu, Lili,Li, Honglin,Xu, Yufang , p. 1507 - 1513 (2019/04/17) |
SHUNYUANSHENG BIO-PHARMTECH CO., LTD is a quality supplier of Crizotinib. Our main goal is customer satisfaction. Contact us to negotiate the best price for your business on Crizotinib 877399-52-5.