Your Location:Home > Products > Organic chemicals
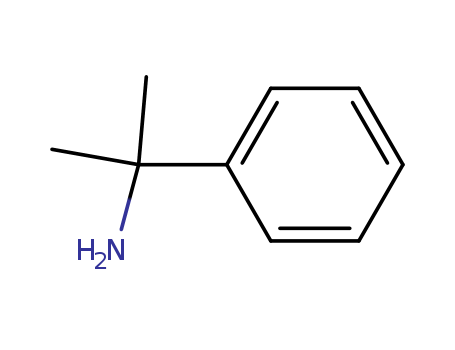

CasNo: 585-32-0
MF: C9H13N
Appearance: Liquid. (Clear, colorless ~ yellow.)
|
Chemical Description |
Cumylamine is an organic compound with the formula C9H13N. |
InChI:InChI=1/C9H13N/c1-9(2,10)8-6-4-3-5-7-8/h3-7H,10H2,1-2H3
Modern photoredox catalysis has traditio...
C-C bond formation by transition metal-c...
Persistent dialkylnitroxides (e.g., 2,2,...
Disclosed is a method for producing a co...
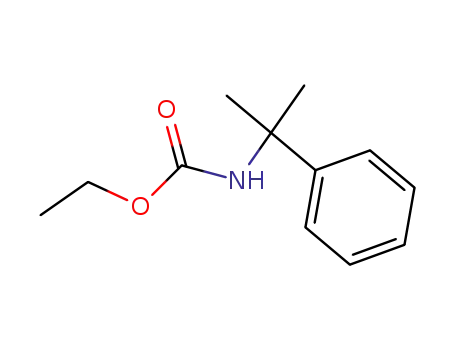
(1-methyl-1-phenyl-ethyl)-carbamic acid ethyl ester

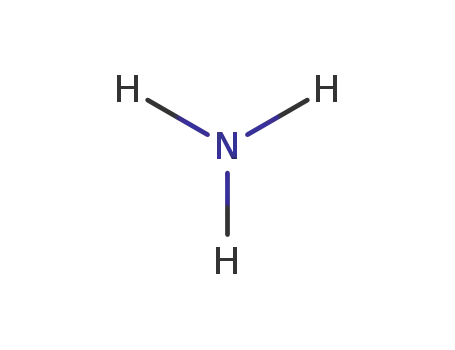
ammonia

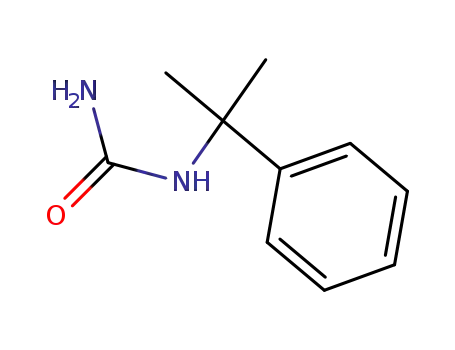
(1-methyl-1-phenyl-ethyl)-urea

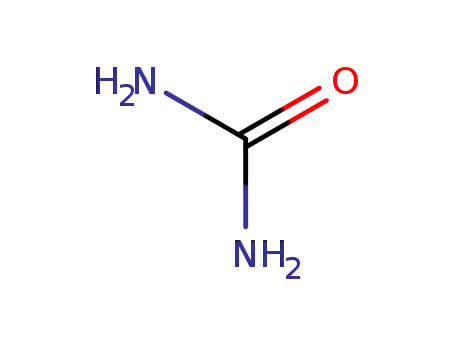
urea

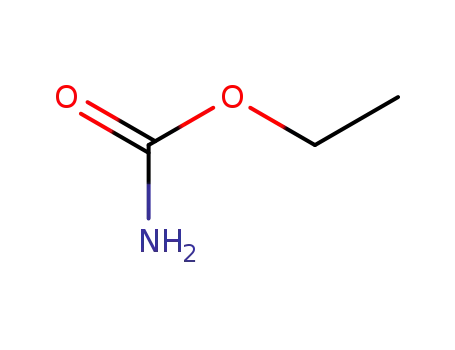
urethane

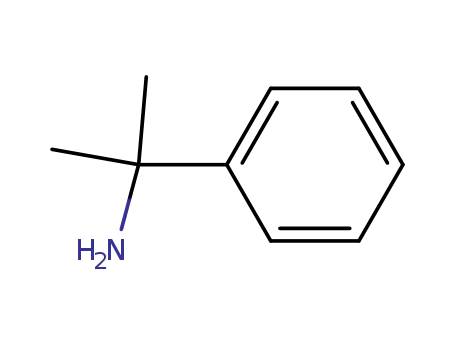
2-phenyl-2-propylamine
| Conditions | Yield |
|---|---|
|
at 180 - 185 ℃;
|
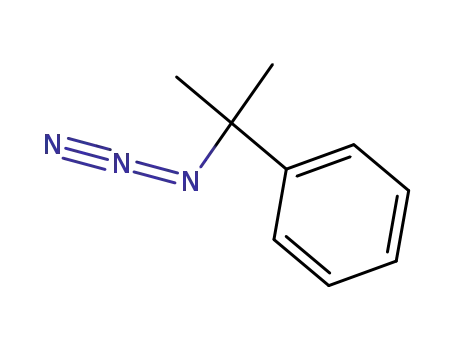
cumyl azide


2-phenyl-2-propylamine
| Conditions | Yield |
|---|---|
|
With
lithium aluminium tetrahydride;
In
diethyl ether;
at 0 - 20 ℃;
for 18h;
|
95% |
|
With
hydrogen;
In
ethanol;
for 12h;
|
94% |
|
With
lithium aluminium tetrahydride;
In
diethyl ether;
1.) 12 h, 2.) reflux, 1 h;
|
68% |
|
With
Raney nickel;
In
isopropyl alcohol;
at 60 - 70 ℃;
|
66% |
|
With
lithium aluminium tetrahydride;
In
diethyl ether;
for 4h;
Heating;
|
61.5% |
|
With
lithium aluminium tetrahydride;
In
diethyl ether;
Heating;
|
|
|
nickel;
In
isopropyl alcohol;
|
|
|
With
lithium aluminium tetrahydride;
In
diethyl ether;
for 3h;
Yield given;
Ambient temperature;
|
|
|
With
sodium chloride;
nickel;
In
ethanol;
Electrolysis;
|
|
|
With
hydrogen;
Lindlar's catalyst;
In
ethanol;
for 12h;
|
19.8 g |
|
With
hydrogen;
Lindlar's catalyst;
In
ethanol;
|
|
|
With
palladium 10% on activated carbon; ammonium formate;
In
ethanol;
at 50 ℃;
|
|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 - 20 ℃;
Inert atmosphere;
|
0.51 g |
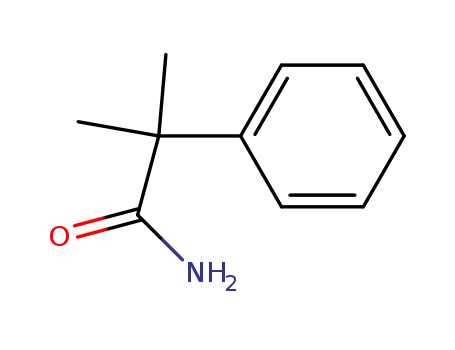
2-methyl-2-phenyl-propanamide
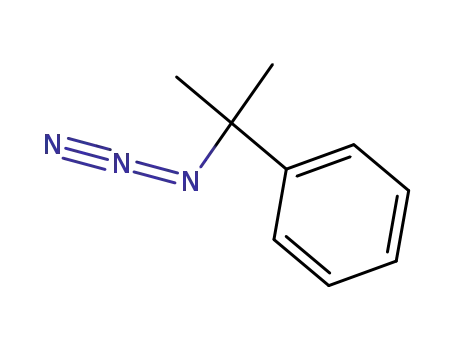
cumyl azide
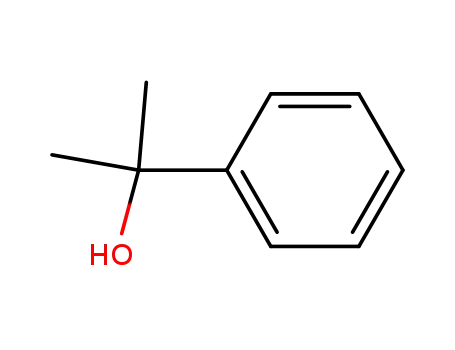
1-methyl-1-phenylethyl alcohol
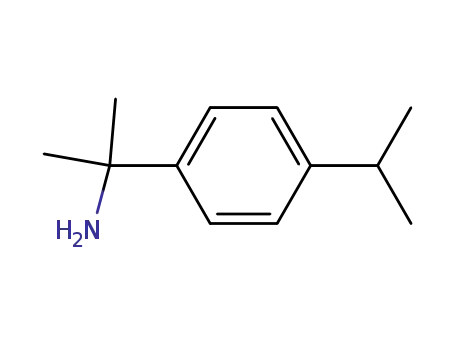
p-(2-Aminoprop-2-yl)-isopropylbenzol

(1-methyl-1-phenyl-ethyl)-carbamic acid ethyl ester
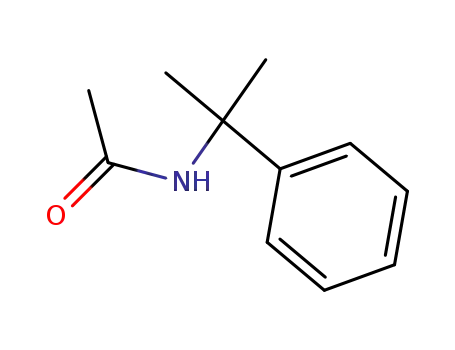
N-(1-methyl-1phenylethyl)acetamide
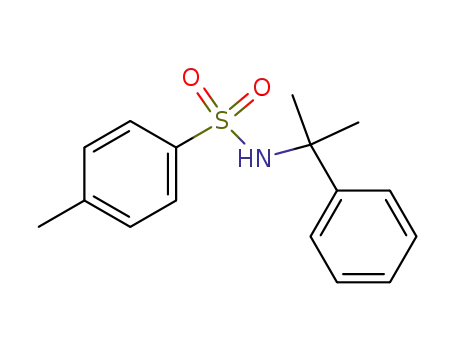
N-(α,α-dimethylbenzyl)-p-toluenesulfonamide
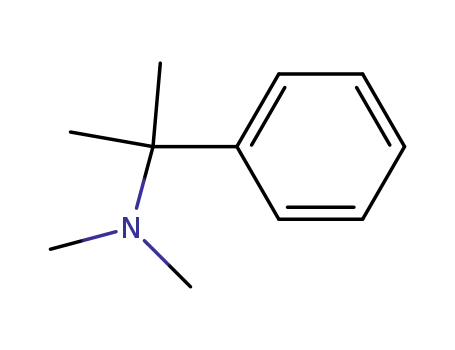
[1-(dimethylamino)-1-methylethyl]benzene