Your Location:Home > Products > Organic chemicals
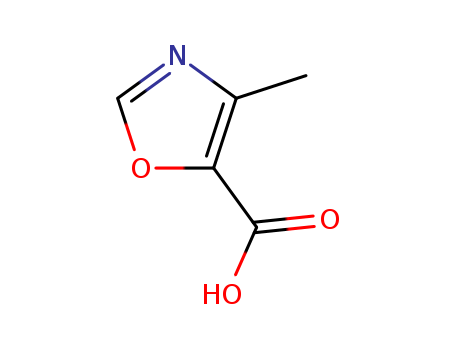

CasNo: 2510-32-9
MF: C5H5NO3
Appearance: white powder
InChI:InChI=1/C5H5NO3/c1-3-4(5(7)8)9-2-6-3/h2H,1H3,(H,7,8)
An approach to synthesis of 2-, 4-, and ...
The disclosure provides compounds of for...
The disclosure provides compounds having...
The disclosure is directed to novel dopa...
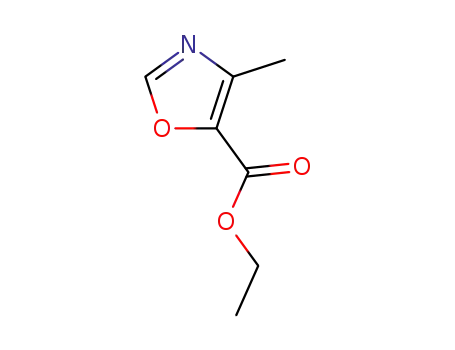
ethyl 4-methyloxazole-5-carboxylate

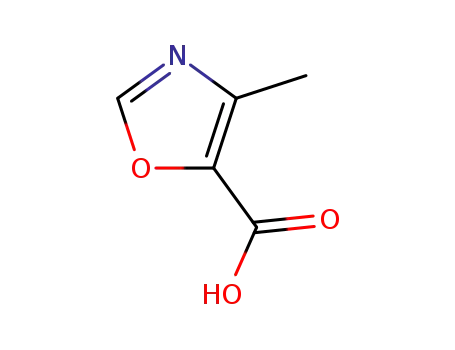
4-methyl-1,3-oxazole-5-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 0 - 25 ℃;
for 2.5h;
|
85% |
|
With
sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 5h;
|
63% |
|
With
sodium hydroxide;
for 0.0166667h;
Heating;
|
6.04 g |
|
ethyl 4-methyloxazole-5-carboxylate;
With
sodium hydroxide; water;
for 0.0166667h;
Heating / reflux;
With
hydrogenchloride;
In
water;
|
|
|
ethyl 4-methyloxazole-5-carboxylate;
With
sodium hydroxide; water;
at 25 ℃;
for 2h;
With
hydrogenchloride; water;
at 0 ℃;
pH=2;
|
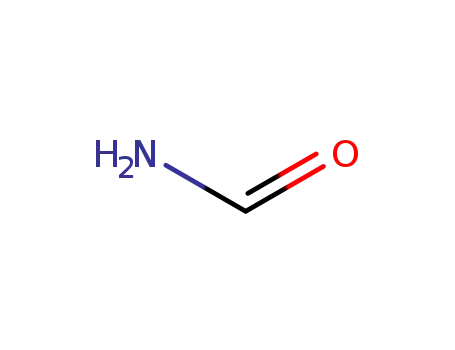
formamide

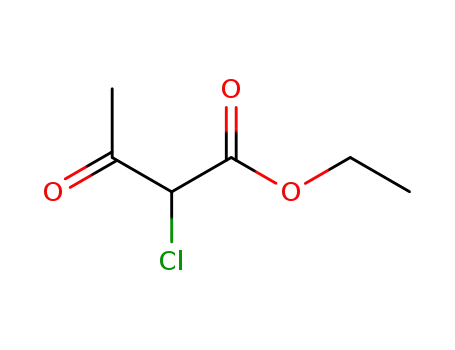
ethyl 2-chloro-3-oxo-butyrate


4-methyl-1,3-oxazole-5-carboxylic acid
| Conditions | Yield |
|---|---|
|
formamide; ethyl 2-chloro-3-oxo-butyrate;
at 20 - 120 ℃;
With
sodium hydroxide; water;
at 20 ℃;
for 4h;
With
hydrogenchloride; water;
pH=2;
|
64.5% |
|
formamide; ethyl 2-chloro-3-oxo-butyrate;
at 20 - 120 ℃;
for 5h;
With
sodium hydroxide; water;
at 20 ℃;
for 4h;
With
hydrogenchloride;
In
water;
pH=2;
|
64.5% |
|
formamide; ethyl 2-chloro-3-oxo-butyrate;
at 20 - 120 ℃;
With
sodium hydroxide; water;
at 20 ℃;
for 4h;
With
hydrogenchloride;
In
water;
pH=2;
Product distribution / selectivity;
|
64.5% |
|
formamide; ethyl 2-chloro-3-oxo-butyrate;
at 20 - 120 ℃;
With
sodium hydroxide;
In
water;
at 20 ℃;
for 4h;
With
hydrogenchloride;
In
water;
pH=2;
|
64.5% |
|
formamide; ethyl 2-chloro-3-oxo-butyrate;
at 20 - 120 ℃;
With
sodium hydroxide;
In
water;
at 20 ℃;
for 4h;
With
hydrogenchloride;
In
water;
pH=2;
|
64.5% |
|
formamide; ethyl 2-chloro-3-oxo-butyrate;
at 20 - 120 ℃;
Inert atmosphere;
With
sodium hydroxide;
at 20 ℃;
for 4h;
Inert atmosphere;
With
hydrogenchloride;
In
water;
pH=2;
Inert atmosphere;
|
44% |
|
formamide; ethyl 2-chloro-3-oxo-butyrate;
at 120 ℃;
for 6h;
Inert atmosphere;
With
water; sodium hydroxide;
at 20 ℃;
for 4h;
|
44% |
|
formamide; ethyl 2-chloro-3-oxo-butyrate;
at 120 ℃;
for 6h;
With
sodium hydroxide;
at 20 ℃;
for 4h;
Inert atmosphere;
|
44% |
|
formamide; ethyl 2-chloro-3-oxo-butyrate;
In
N,N-dimethyl-formamide;
at 120 ℃;
for 21h;
With
sodium hydroxide; water;
In
tert-butyl methyl ether; N,N-dimethyl-formamide;
at 20 ℃;
for 3h;
With
hydrogenchloride;
In
water;
at 20 ℃;
for 2.16667h;
pH=2;
Product distribution / selectivity;
|
35.3% |
|
formamide; ethyl 2-chloro-3-oxo-butyrate;
In
N,N-dimethyl-formamide;
at 120 ℃;
for 21h;
With
sodium hydroxide;
In
water; N,N-dimethyl-formamide;
at 20 ℃;
for 3h;
With
hydrogenchloride;
In
water; N,N-dimethyl-formamide;
at 20 ℃;
for 2.16667h;
pH=2;
|
35.5% |

formamide
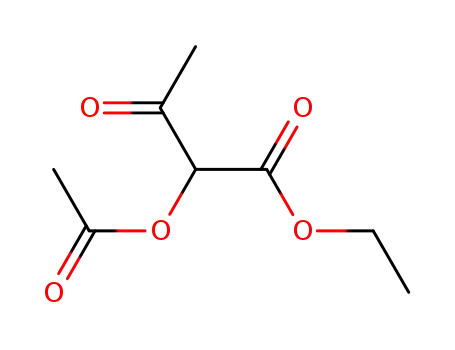
2-acetoxy-acetoacetic acid ethyl ester

ethyl 2-chloro-3-oxo-butyrate

ethyl 4-methyloxazole-5-carboxylate
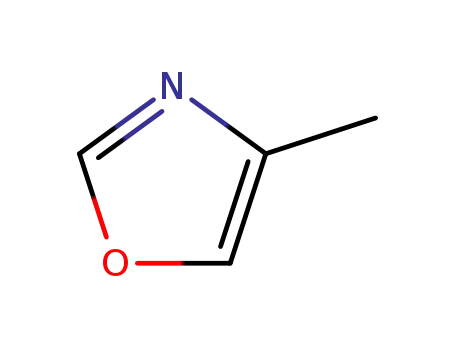
4-methyloxazole
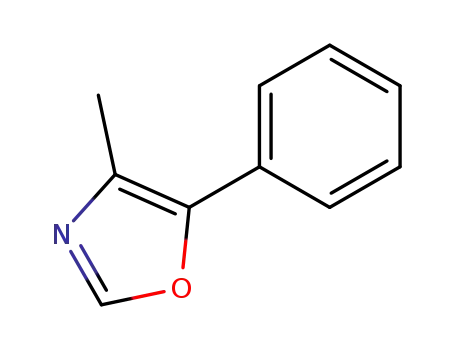
4-methyl-5-phenyl-1,3-oxazole
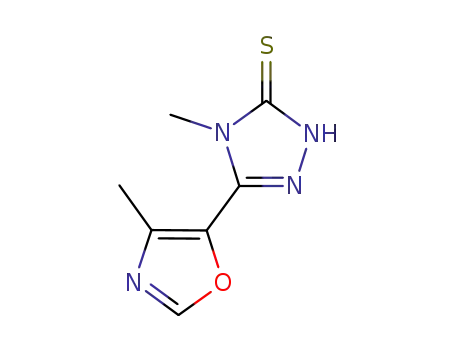
5-(4-methyl-5-oxazoyl)-4-methyl-2,4-dihydro-3H-1,2,4-triazole-3-thione
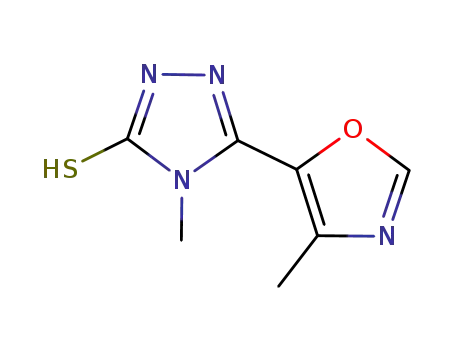
4-methyl-5-(4-methyl-1,3-oxazol-5-yl)-4H-1,2,4-triazole-3-thiol