Your Location:Home > Products > Pharmaceutical Intermediates
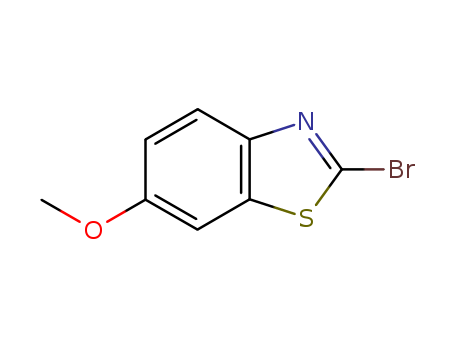

CasNo: 2941-58-4
MF: C8H6 Br N O S
|
Synthesis |
2-Bromo-6-methoxybenzo[d]thiazole (B2) (Scheme 1). 6- Methoxybenzo[d]thiazol-2-amine (5.0 g; 27.7 mmol) was dissolved in 125 mL CHBCN. t- Butylnitrite (3.4 mL; 28 mmol) was added slowly. CuBr (5.0 g; 34.9 mmol) was added portion-wise through a funnel. The reaction was monitored by HPLC. After 3 hours, ethyl acetate (500 mL) was added, and the mixture was filtered through celite. The organic layer was washed with brine twice (200 mL each). The organic layer was dried over MgSO4. After filtration, the solvent was removed. The residual was dissolved in 50 mL CH2Cl2, and 2 g of silica gel was added. After drying, the silica gel loaded on a silica gel cake in hexane. The product was eluted with 5% ethyl acetate and 95% hexane. A yellowish band was collected (1.5 L). The solvent was removed to afford the product. Yield: 0.81 g 12%. |
InChI:InChI=1/C8H6BrNOS/c1-11-5-2-3-6-7(4-5)12-8(9)10-6/h2-4H,1H3
The present invention provides an α-synu...
Bioluminescence imaging with luciferase-...
Disclosed are compounds of Formula (I) t...
PROBLEM TO BE SOLVED: To provide a metho...
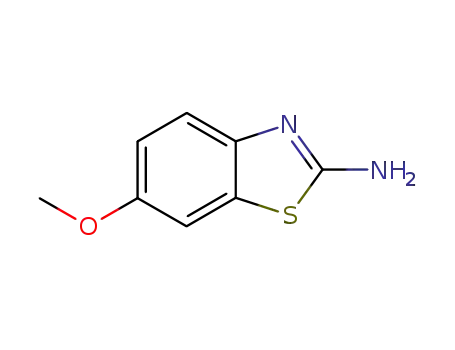
6-methoxybenzothiazol-2-ylamine

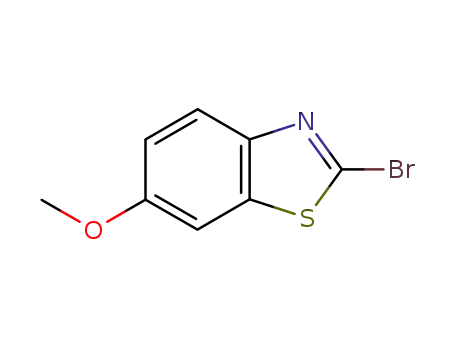
2-bromo-6-methoxy-1,3 benzothiazole
| Conditions | Yield |
|---|---|
|
With
copper(ll) bromide; isopentyl nitrite;
In
acetonitrile;
at 65 ℃;
|
95% |
|
With
nitrous acid isobutyl ester; copper(ll) bromide;
In
acetonitrile;
at 65 ℃;
for 4h;
Inert atmosphere;
|
95% |
|
With
tert.-butylnitrite; copper(ll) bromide;
In
acetonitrile;
at 80 ℃;
for 2h;
|
89% |
|
With
tert.-butylnitrite; tetrabutylammomium bromide; toluene-4-sulfonic acid; copper(ll) bromide;
In
water; acetonitrile;
at 20 ℃;
for 1h;
Inert atmosphere;
|
87.1% |
|
With
tert.-butylnitrite; copper(ll) bromide;
In
acetonitrile;
|
87.5% |
|
With
tert.-butylnitrite; copper(ll) bromide;
In
acetonitrile;
|
87.5% |
|
With
tert.-butylnitrite; copper(ll) bromide;
In
acetonitrile;
at 80 ℃;
for 2h;
|
85% |
|
With
tert.-butylnitrite; copper(ll) bromide;
In
acetonitrile;
at 0 - 20 ℃;
|
84% |
|
With
polyethylene glycol; copper(ll) bromide; isopentyl nitrite;
In
acetonitrile;
at 50 ℃;
for 3h;
|
79.3% |
|
With
PEG 200; copper(ll) bromide; isopentyl nitrite;
In
acetonitrile;
at 65 ℃;
for 3h;
|
79.3% |
|
With
copper(I) bromide; isopentyl nitrite;
In
acetonitrile;
at 65 ℃;
for 3h;
Inert atmosphere;
|
78% |
|
With
copper(ll) bromide; isopentyl nitrite;
|
65% |
|
With
tert.-butylnitrite; copper(ll) bromide;
In
acetonitrile;
for 3h;
|
62% |
|
With
tetrabutylammomium bromide; toluene-4-sulfonic acid; copper(ll) bromide; isopentyl nitrite;
In
acetonitrile;
at 25 ℃;
for 0.75h;
Inert atmosphere;
|
52% |
|
With
tert.-butylnitrite; copper(I) bromide;
In
acetonitrile;
for 3h;
|
12% |
|
|

6-methoxybenzothiazol-2-ylamine


copper(I) bromide


2-bromo-6-methoxy-1,3 benzothiazole
| Conditions | Yield |
|---|---|
|
With
sodium nitrite;
In
water; hydrogen bromide;
|

6-methoxybenzothiazol-2-ylamine

copper(I) bromide
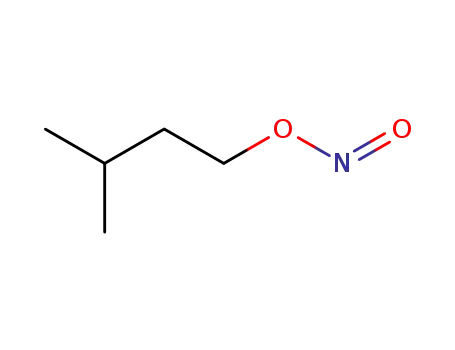
isopentyl nitrite
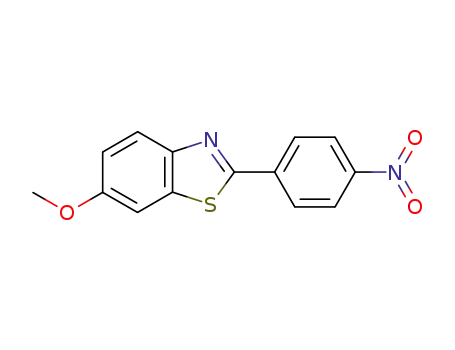
6-methoxy-2-(4-nitrophenyl)benzothiazole
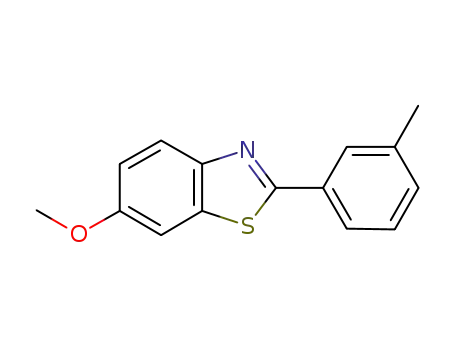
6-methoxy-2-m-tolylbenzo[d]thiazole
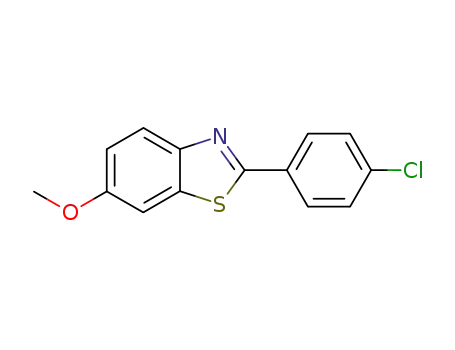
2-(4-chlorophenyl)-6-methoxybenzo[d]thiazole
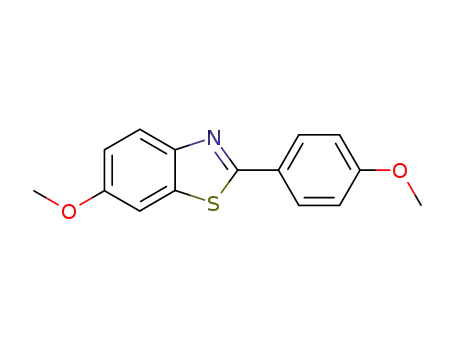
6-methoxy-2-(4-methoxyphenyl)benzo[d]thiazole