Your Location:Home > Products > Organic chemicals
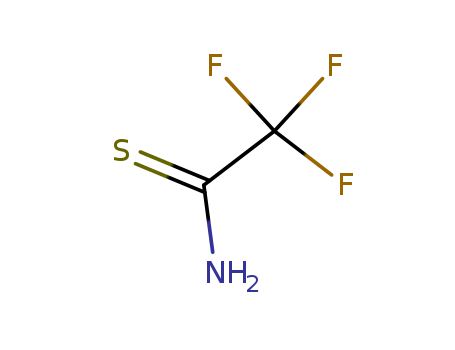

CasNo: 421-52-3
MF: C2H2 F3 N S
|
General Description |
2,2,2-trifluoroethanethioamide, also known as trifluoroethyl thioacetamide, is a chemical compound with the molecular formula C4H6F3NS. It is a thioamide derivative with three fluorine atoms attached to the carbon atom and a sulfur atom bonded to the nitrogen atom. 2,2,2-trifluoroethanethioamide is used in organic synthesis as a source of nucleophilic 2,2,2-trifluoroethylthio anion, which can participate in a variety of reactions. Additionally, it is also used as a building block for the synthesis of pharmaceuticals and agrochemicals. This chemical has a wide range of industrial applications due to its unique chemical properties and reactivity. |
InChI:InChI=1/C2H2F3NS/c3-2(4,5)1(6)7/h(H2,6,7)
Polyfluoroalkyldithiocarboxylates react ...
The present invention relates to a compo...
There is herein provided a compound of f...
The invention relates to oxazole and thi...
The invention is directed to certain nov...
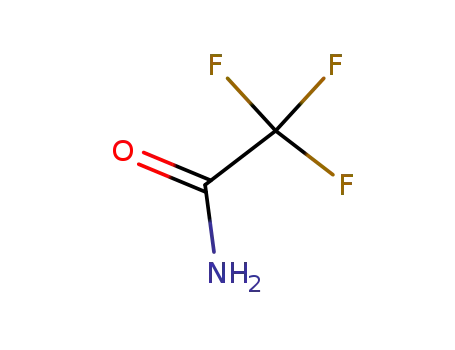
2,2,2-trifluoroacetamide

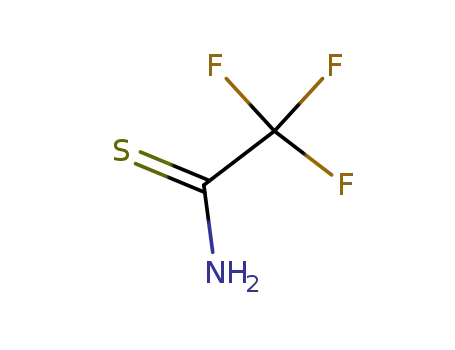
trifluorothioacetamide
| Conditions | Yield |
|---|---|
|
With
tetraphosphorus decasulfide;
In
tetrahydrofuran;
at 70 - 75 ℃;
for 4h;
Inert atmosphere;
|
95% |
|
With
Lawessons reagent;
In
tetrahydrofuran;
for 2h;
Heating / reflux;
|
84% |
|
With
Lawessons reagent;
In
tetrahydrofuran;
for 2h;
Reflux;
|
65% |
|
With
Lawessons reagent;
In
tetrahydrofuran;
for 6h;
Heating;
|
53% |
|
With
tetraphosphorus decasulfide; Hexamethyldisiloxane;
In
toluene;
at 45 ℃;
for 15h;
|
44% |
|
With
P2S2;
In
benzene;
for 96h;
Heating;
|
|
|
With
Lawessons reagent;
|
|
|
With
Lawessons reagent;
In
tetrahydrofuran;
for 18h;
Heating / reflux;
|
|
|
With
Lawessons reagent;
In
tetrahydrofuran;
for 6h;
Reflux;
|
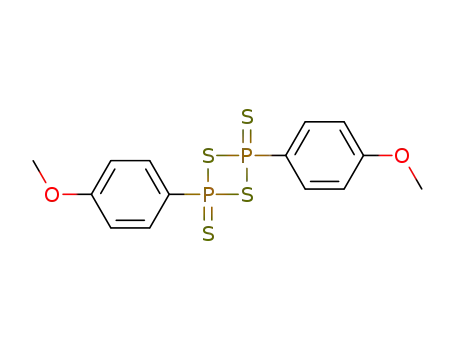
Lawessons reagent


2,2,2-trifluoroacetamide


trifluorothioacetamide
| Conditions | Yield |
|---|---|
|
|
84% |
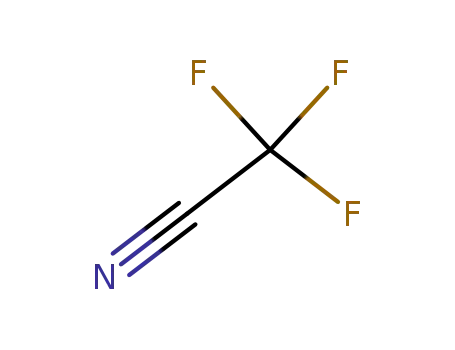
trifluoroacetonitrile

2,2,2-trifluoroacetamide

Lawessons reagent
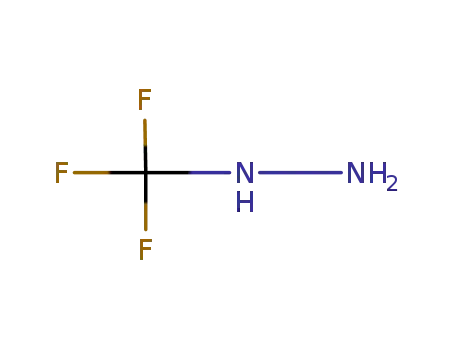
trifluoromethylhydrazine
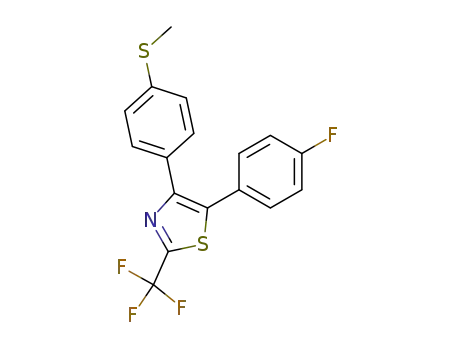
5-(4-fluorophenyl)-4-(4-methylthiophenyl)-2-trifluoromethylthiazole
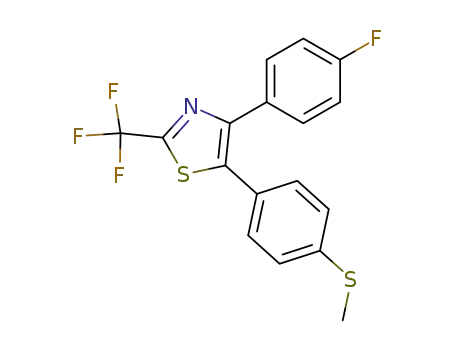
4-(4-fluorophenyl)-5-(4-methylthiophenyl)-2-trifluoromethylthiazole
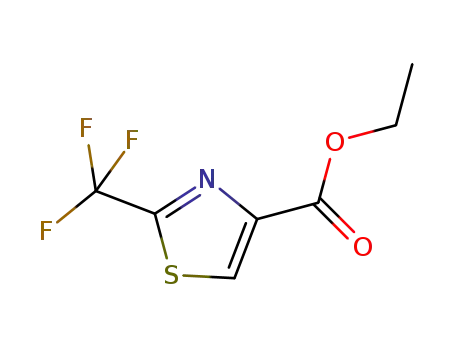
Ethyl 2-(trifluoromethyl)thiazole-4-carboxylate
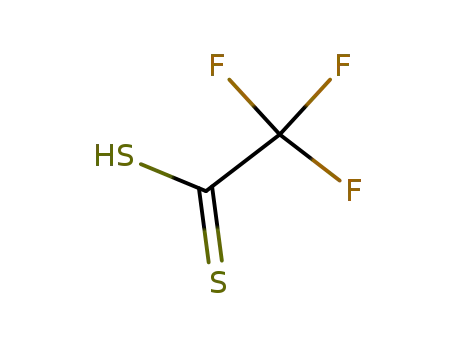
Trifluordithio-essigsaeure