Your Location:Home > Products > Pharmaceutical Intermediates
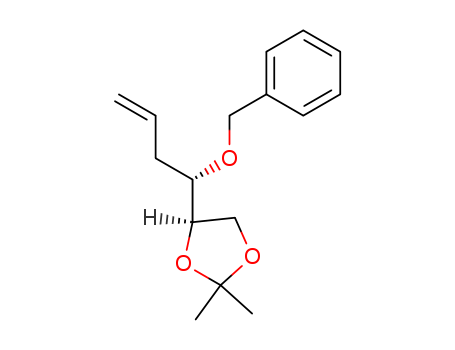

CasNo: 87604-53-3
The invention belongs to the field of ch...
Two new [named xylapyrrosides A (1) and ...
The stereoselective total synthesis of (...
Synthetic routes to (S)-oxiracetam and (...
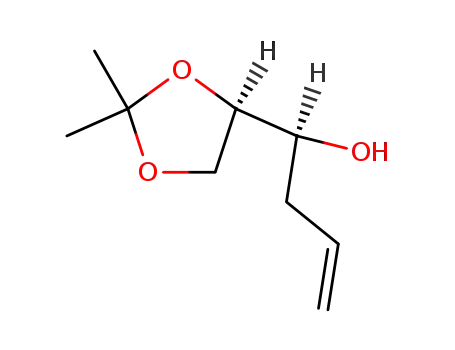
(S)-1-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)but-3-en-1-ol

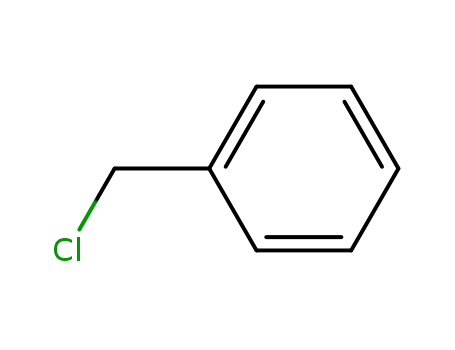
benzyl chloride

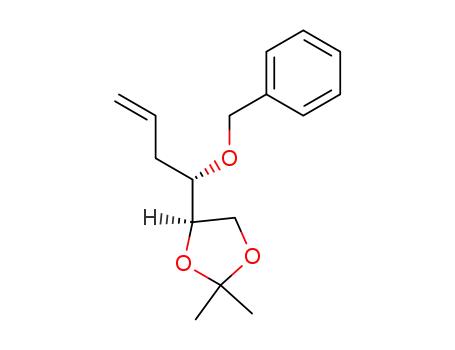
(R)-4-((S)-1-(benzyloxy)but-3-ene-1-yl)-2,2-dimethyl-1,3-dioxypentane
| Conditions | Yield |
|---|---|
|
(S)-1-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)but-3-en-1-ol;
With
sodium hydride;
In
N,N-dimethyl-formamide;
at 0 ℃;
for 0.5h;
benzyl chloride;
In
N,N-dimethyl-formamide;
at 0 - 5 ℃;
for 1h;
|
75% |
|
With
sodium hydride;
Yield given. Multistep reaction;
1.) THF, DMSO, 2h, reflux 2.) 2h, RT;
|
|
|
With
1H-imidazole; sodium hydride;
In
N,N-dimethyl-formamide;
|

(S)-1-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)but-3-en-1-ol

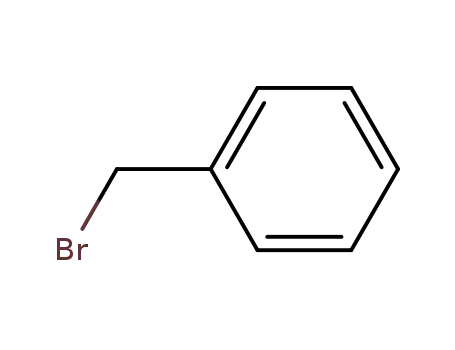
benzyl bromide


(R)-4-((S)-1-(benzyloxy)but-3-ene-1-yl)-2,2-dimethyl-1,3-dioxypentane
| Conditions | Yield |
|---|---|
|
With
sodium hydride;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
for 6h;
|
94% |
|
With
sodium hydride;
In
tetrahydrofuran; mineral oil;
at 0 - 20 ℃;
for 5h;
Inert atmosphere;
|
94% |
|
With
sodium hydride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 5h;
|
92% |
|
With
sodium hydride;
In
tetrahydrofuran; mineral oil;
at 0 - 20 ℃;
for 4h;
Inert atmosphere;
|
92% |
|
With
sodium hydride;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
for 12.5h;
|
90% |
|
(S)-1-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)but-3-en-1-ol;
With
sodium hydride;
In
tetrahydrofuran;
at 0 ℃;
for 0.333333h;
Inert atmosphere;
benzyl bromide;
In
tetrahydrofuran;
at 20 ℃;
for 4h;
Inert atmosphere;
|
90% |
|
With
sodium hydride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 16h;
|
85% |
|
With
tetra-(n-butyl)ammonium iodide; sodium hydride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 2h;
|
85% |
|
(S)-1-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)but-3-en-1-ol;
With
sodium hydride;
In
N,N-dimethyl-formamide; mineral oil;
at 0 ℃;
for 2h;
Inert atmosphere;
benzyl bromide;
In
N,N-dimethyl-formamide; mineral oil;
at 0 - 20 ℃;
for 14h;
Inert atmosphere;
|
85% |
|
(S)-1-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)but-3-en-1-ol;
With
sodium hydride;
In
N,N-dimethyl-formamide;
at 0 ℃;
for 0.5h;
benzyl bromide;
In
N,N-dimethyl-formamide;
at 0 - 5 ℃;
for 1h;
|
80% |
|
With
sodium hydride;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 3h;
|
65% |
|
With
sodium hydride;
Yield given. Multistep reaction;
1) DMF, 0 deg C, 15 min, 2) 23 deg C, 2h;
|

(S)-1-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)but-3-en-1-ol
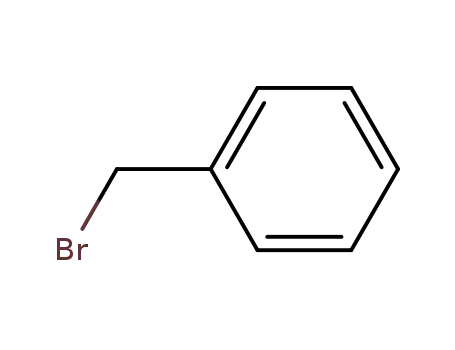
benzyl bromide
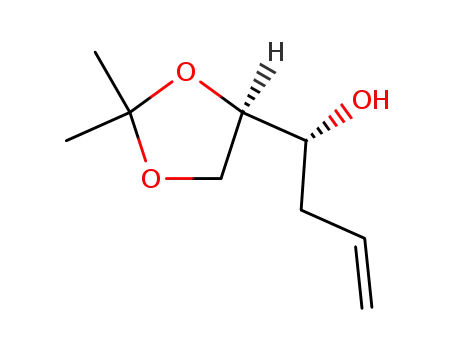
(2R,3R)-1,2-O-isopropylidene-5-hexene-1,2,3-triol
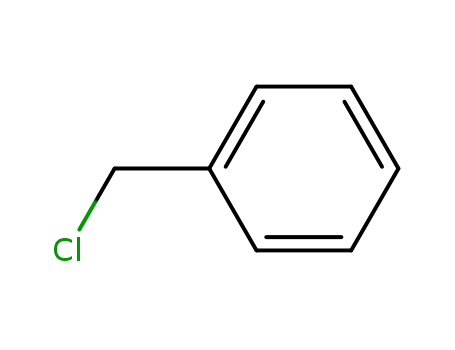
benzyl chloride
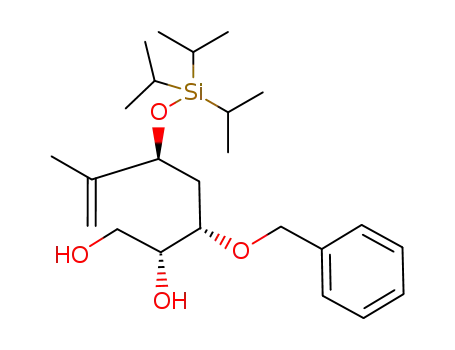
(2R,3S,5S)-3-Benzyloxy-6-methyl-5-triisopropylsilanyloxy-hept-6-ene-1,2-diol
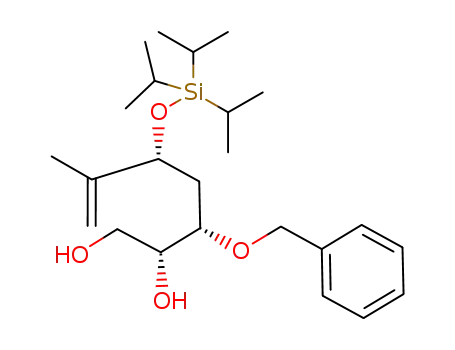
(2R,3S,5R)-3-Benzyloxy-6-methyl-5-triisopropylsilanyloxy-hept-6-ene-1,2-diol
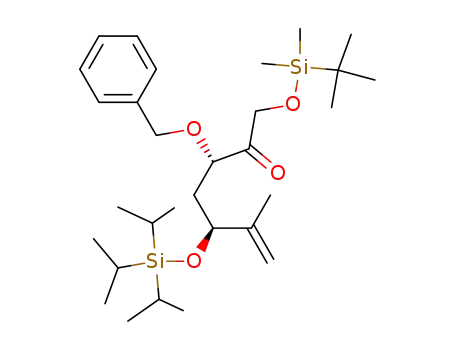
(3S,5S)-3-Benzyloxy-1-(tert-butyl-dimethyl-silanyloxy)-6-methyl-5-triisopropylsilanyloxy-hept-6-en-2-one
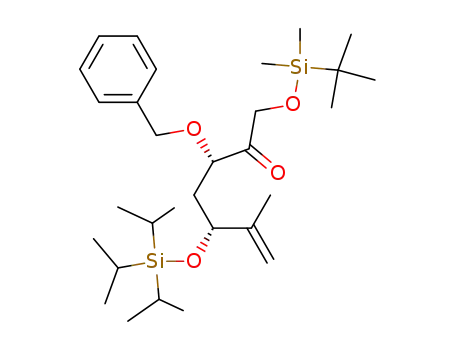
(3S,5R)-3-Benzyloxy-1-(tert-butyl-dimethyl-silanyloxy)-6-methyl-5-triisopropylsilanyloxy-hept-6-en-2-one