Your Location:Home > Products > Pharmaceutical Intermediates
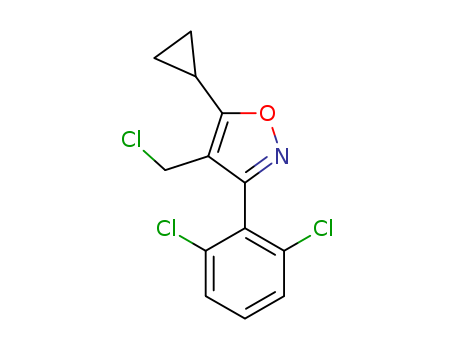

CasNo: 1268245-50-6
The invention relates to activators of F...
The present disclosure disclosed a modul...
The disclosure relates to activators of ...
The present technology is directed to co...
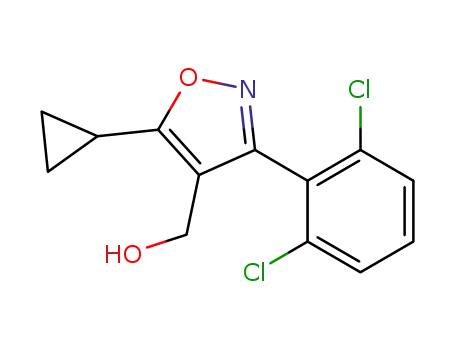
4-(hydroxymethyl)-5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazole

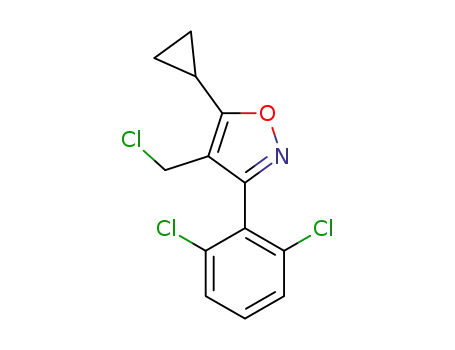
4-(chloromethyl)-5-cyclopropyl-3-(2,6-dichlorophenyl)-1,2-oxazole
| Conditions | Yield |
|---|---|
|
With
thionyl chloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 20 ℃;
for 1h;
|
94% |
|
With
thionyl chloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 20 ℃;
for 1h;
|
91% |
|
4-(hydroxymethyl)-5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazole;
With
1,2,3-Benzotriazole; thionyl chloride;
In
dichloromethane;
at 0 - 20 ℃;
With
sodium hydroxide;
In
dichloromethane; water;
|
81% |
|
With
1,2,3-Benzotriazole; thionyl chloride;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
|
81% |
|
With
1,2,3-Benzotriazole; thionyl chloride;
In
dichloromethane;
at 0 - 25 ℃;
for 1.5h;
|
80% |
|
With
1,2,3-Benzotriazole; thionyl chloride;
In
dichloromethane;
at 0 - 20 ℃;
|
79% |
|
With
1,2,3-Benzotriazole; thionyl chloride;
In
dichloromethane;
at 0 - 20 ℃;
|
79% |
|
With
thionyl chloride;
In
4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran;
at 20 ℃;
for 2h;
|
|
|
With
thionyl chloride;
In
dichloromethane;
at 20 ℃;
for 1.5h;
|
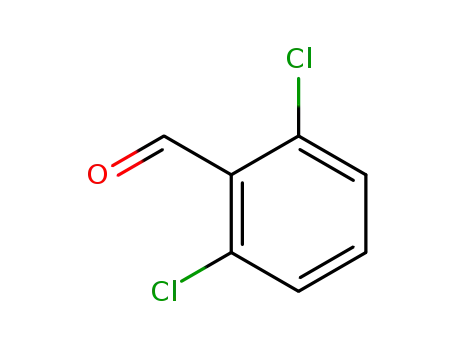
2,6-dichlorobenzaldehyde


4-(chloromethyl)-5-cyclopropyl-3-(2,6-dichlorophenyl)-1,2-oxazole
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1: hydroxylamine hydrochloride; sodium hydroxide / ethanol; water / 24 h / 90 °C
2: N-chloro-succinimide / N,N-dimethyl-formamide / 1 h / 20 °C
3: triethylamine / 20 °C
4: diisobutylaluminium hydride / tetrahydrofuran / 0 - 20 °C / Inert atmosphere
5: thionyl chloride; 1,2,3-Benzotriazole / dichloromethane / 0 - 20 °C
With
1,2,3-Benzotriazole; N-chloro-succinimide; thionyl chloride; hydroxylamine hydrochloride; diisobutylaluminium hydride; triethylamine; sodium hydroxide;
In
tetrahydrofuran; ethanol; dichloromethane; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1: hydroxylamine hydrochloride; sodium hydroxide / ethanol; water / 18 - 24 h / 80 - 90 °C
2: N-chloro-succinimide / N,N-dimethyl-formamide / 20 °C
3: triethylamine / 20 °C
4: diisobutylaluminium hydride / tetrahydrofuran / 0 - 20 °C / Inert atmosphere
5: thionyl chloride; 1,2,3-Benzotriazole / dichloromethane / 0 - 20 °C / Inert atmosphere
With
1,2,3-Benzotriazole; N-chloro-succinimide; thionyl chloride; hydroxylamine hydrochloride; diisobutylaluminium hydride; triethylamine; sodium hydroxide;
In
tetrahydrofuran; ethanol; dichloromethane; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1: sodium hydroxide; hydroxylamine hydrochloride / water; ethanol / 0 - 90 °C
2: N-chloro-succinimide / N,N-dimethyl-formamide / 2 h / 20 °C
3: triethylamine / 16 h / 20 °C
4: lithium aluminium tetrahydride / tetrahydrofuran / 2 h / 0 - 20 °C
5: thionyl chloride; 1,2,3-Benzotriazole / dichloromethane / 0 - 20 °C
With
1,2,3-Benzotriazole; N-chloro-succinimide; lithium aluminium tetrahydride; thionyl chloride; hydroxylamine hydrochloride; triethylamine; sodium hydroxide;
In
tetrahydrofuran; ethanol; dichloromethane; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1: hydroxylamine hydrochloride; sodium hydroxide / ethanol / 90 °C
2: N-chloro-succinimide / N,N-dimethyl-formamide / 1 h / 20 °C
3: triethylamine / tetrahydrofuran / 20 °C
4: lithium aluminium tetrahydride / tetrahydrofuran / 2 h / 0 °C / Inert atmosphere
5: thionyl chloride; N,N-dimethyl-formamide / dichloromethane / 1 h / 20 °C
With
N-chloro-succinimide; lithium aluminium tetrahydride; thionyl chloride; hydroxylamine hydrochloride; triethylamine; N,N-dimethyl-formamide; sodium hydroxide;
In
tetrahydrofuran; ethanol; dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1: hydroxylamine hydrochloride; sodium hydroxide / water; ethanol / 0 - 90 °C
2: N-chloro-succinimide / N,N-dimethyl-formamide / 2 h / 20 °C
3: triethylamine / 16 h / 20 °C
4: lithium aluminium tetrahydride / tetrahydrofuran / 2 h / 0 - 20 °C
5: 1,2,3-Benzotriazole; thionyl chloride / dichloromethane / 0 - 20 °C
With
1,2,3-Benzotriazole; N-chloro-succinimide; lithium aluminium tetrahydride; thionyl chloride; hydroxylamine hydrochloride; triethylamine; sodium hydroxide;
In
tetrahydrofuran; ethanol; dichloromethane; water; N,N-dimethyl-formamide;
|
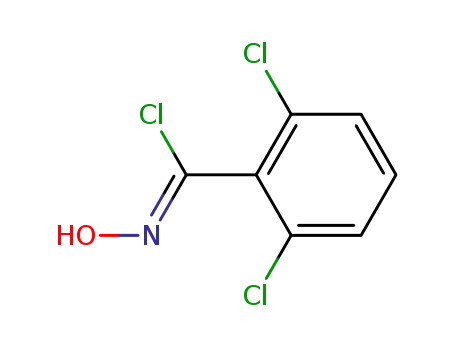
2,6-Dichloro-N-hydroxybenzenecarboximidoyl chloride

2,6-dichlorobenzaldehyde

4-(hydroxymethyl)-5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazole
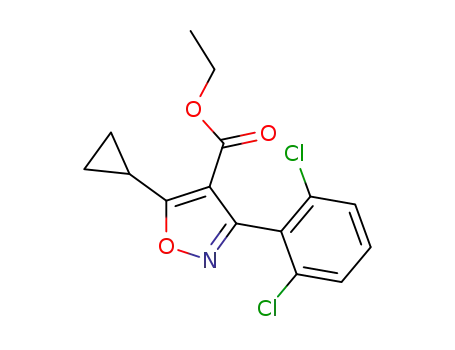
ethyl 5-cyclopropyl-3-(2,6-dichlorophenyl)-1,2-oxazole-4-carboxylate
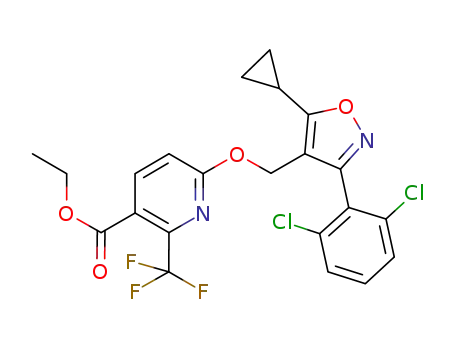
C22H17Cl2F3N2O4
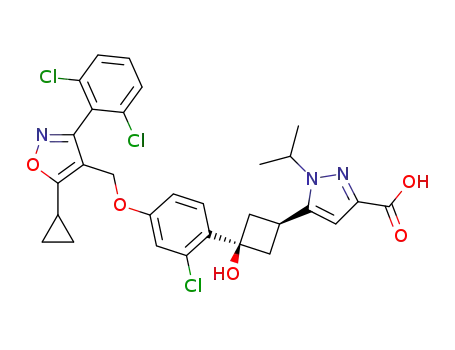
5-((1s,3s)-3-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)phenyl)-3-hydroxycyclobutyl)-1-isopropyl-1H-pyrazole-3-carboxylic acid
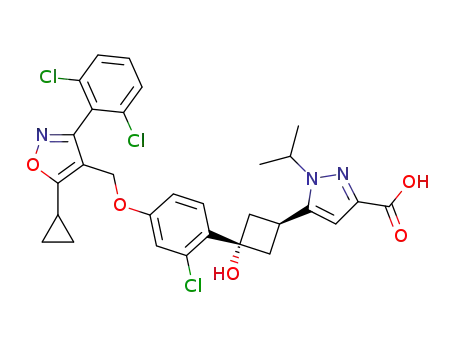
5-((1r,3r)-3-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)phenyl)-3-hydroxycyclobutyl)-1-isopropyl-1H-pyrazole-3-carboxylate
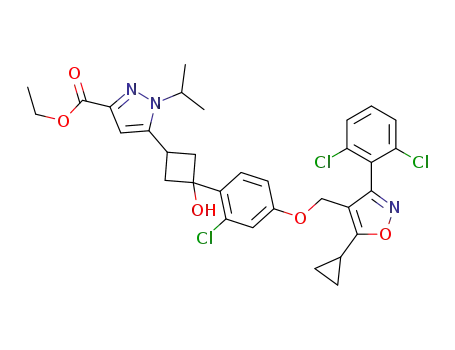
ethyl 5-((1s,3s)-3-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)phenyl)-3-hydroxycyclobutyl)-1-isopropyl-1H-pyrazole-3-carboxylate