Your Location:Home > Products > Organic chemicals
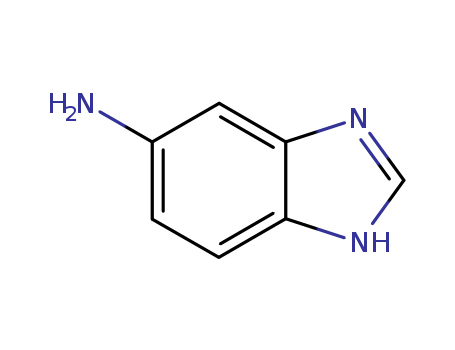

CasNo: 934-22-5
MF: C7H7N3
|
General Description |
1H-Benzimidazol-5-ylamine, also known as 5-Aminobenzimidazole, is an organic compound with the formula C7H7N3. It is a heterocyclic aromatic amine containing both an imidazole and an aniline functional group. This chemical is commonly used as a building block in the synthesis of pharmaceuticals, agrochemicals, and dyes. It has also been studied for its potential as an anti-cancer agent. 1H-Benzimidazol-5-ylamine is a white to off-white crystalline solid at room temperature and is soluble in polar organic solvents such as ethanol and dimethyl sulfoxide, while being only sparingly soluble in water. It is important to handle this chemical with caution as it can cause skin and eye irritation, and proper safety measures should be taken when working with it. |
InChI:InChI=1/C7H7N3/c8-5-1-2-6-7(3-5)10-4-9-6/h1-4H,8H2,(H,9,10)
A new Co(II) three-dimensional coordinat...
The development of green and efficient m...
We report an efficient catalytic protoco...
A series of thieno[2,3-d]pyrimidine-base...
Schistosomiasis is one of the neglected ...
5-nitrobenzimidazole

5-aminobenzimidazole
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium 10% on activated carbon;
In
methanol;
for 20h;
under 760.051 Torr;
|
100% |
|
With
hydrogen;
palladium 10% on activated carbon;
In
tetrahydrofuran; ethanol;
at 20 ℃;
for 4h;
|
92% |
|
With
hydrazine hydrate;
In
ethanol;
at 75 ℃;
for 12h;
Inert atmosphere;
|
88% |
|
With
[Co(κS,N-4-(trifluoromethyl)pyrimidine-2-thiolate)3]; methylhydrazine;
In
methanol;
at 70 ℃;
for 18h;
Sealed tube;
|
86% |
|
With
methanol; palladium on activated charcoal;
at 20 ℃;
for 16h;
|
85% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 20 ℃;
for 6h;
under 45004.5 Torr;
|
85% |
|
With
palladium on activated charcoal; hydrazine hydrate;
In
pentan-1-ol;
at 110 ℃;
|
77% |
|
With
iron; acetic acid;
In
ethanol; water;
Heating;
|
71% |
|
With
hydrogenchloride; iron(II) sulfate;
|
|
|
With
hydrogenchloride; tin(ll) chloride;
|
|
|
With
ethanol; nickel;
Hydrogenation;
|
|
|
With
hydrogen;
nickel;
In
methanol;
under 900.07 Torr;
|
|
|
palladium;
In
tetrahydrofuran;
|
|
|
With
hydrazine hydrate;
In
tetrahydrofuran;
at 100 ℃;
for 10h;
chemoselective reaction;
|
99 %Chromat. |
|
With
hydrogen;
palladium 10% on activated carbon;
In
tetrahydrofuran; methanol;
at 20 ℃;
for 3h;
under 22502.3 Torr;
|
|
|
With
palladium on activated charcoal; hydrazine hydrate;
In
ethanol;
for 4h;
Reflux;
|
0.163 g |
|
With
tin(II) chloride dihdyrate;
In
ethyl acetate;
for 12h;
Reflux;
|
|
|
With
hydrogen;
In
methanol;
at 100 ℃;
for 8h;
under 7500.75 Torr;
chemoselective reaction;
Autoclave;
|
99 %Chromat. |
|
With
sodium tetrahydroborate;
In
water;
at 20 ℃;
for 0.0388889h;
|
|
|
With
sodium tetrahydroborate;
In
water;
at 20 ℃;
for 0.05h;
chemoselective reaction;
|
formic acid

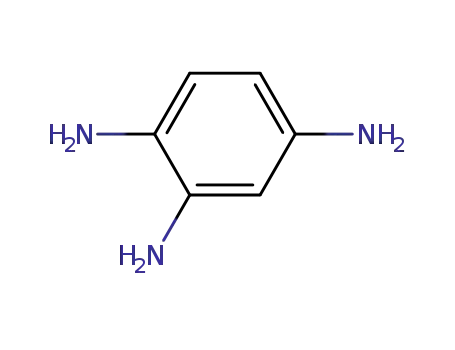
benzene-1,2,4-triamine


5-aminobenzimidazole
| Conditions | Yield |
|---|---|
|
With
tetrabutyl-ammonium chloride;
In
water; toluene;
at 160 ℃;
for 0.166667h;
Microwave irradiation;
|
81% |
|
With
chloro-trimethyl-silane;
In
water; N,N-dimethyl-formamide;
at 120 ℃;
for 0.166667h;
Microwave irradiation;
|
81% |
|
Behandeln der entstandenen Formylverbindung mit siedender verduennter Schwefelsaeure;
|
|
|
anschliessend Behandeln mit verd.Schwefelsaeue;
|

formic acid

benzene-1,2,4-triamine

5-nitrobenzimidazole
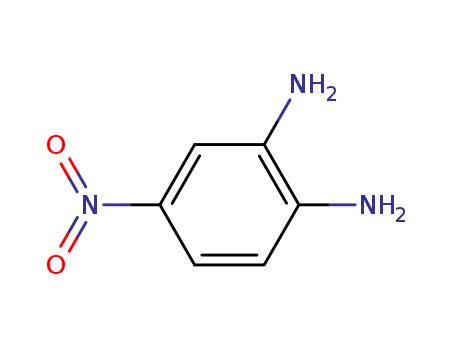
4-Nitrophenylene-1,2-diamine
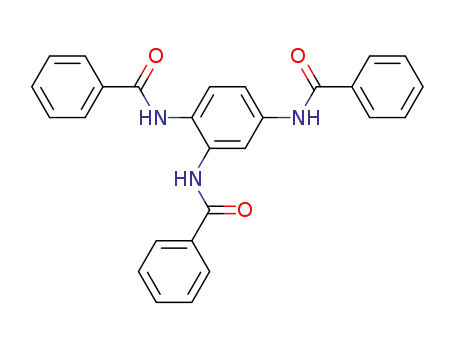
N,N',N''-benzene-1,2,4-triyl-tris-benzamide
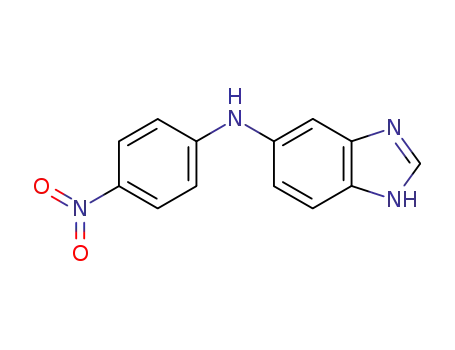
5(6)-N-(4-Nitrophenyl)-aminobenzimidazole
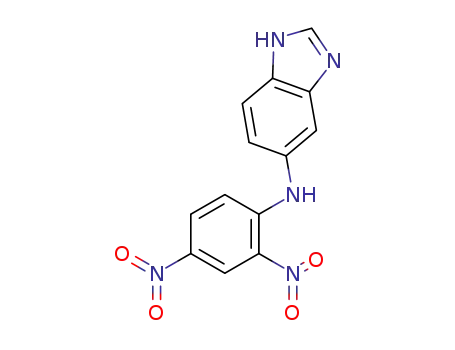
5(6)-N-(2,4-Dinitrophenyl)-aminobenzimidazole
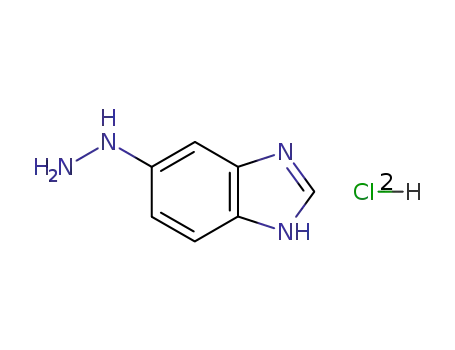
5-hydrazinobenzimidazole dihydrochloride