Your Location:Home > Products > Pharmaceutical Intermediates
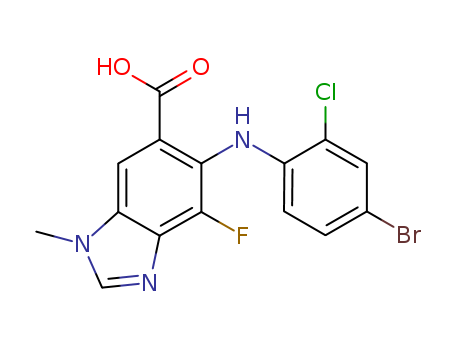

CasNo: 606144-04-1
MF: C15H10BrClFN3O2
The invention relates to a method for pr...
The invention relates to a synthesis met...
The invention provides a substituted ben...
The present invention relates to interme...
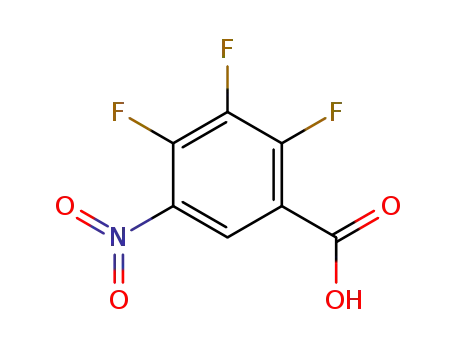
2,3,4-trifluoro-5-nitro-benzoic acid

![5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-1-methyl-1H-benzimidazole-6-carboxylic acid](/upload/2025/5/6ac43359-42b7-48b4-a206-37032d7b5a53.png)
5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-1-methyl-1H-benzimidazole-6-carboxylic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 9 steps
1: ammonium hydroxide / water / 6 h / 0 °C
2: methanol / 1 h / 0 °C
3: 5,5-dimethyl-1,3-cyclohexadiene / 10 h / 125 °C
4: ammonium chloride; iron / water; ethanol / 0.5 h / 70 °C
5: ethanol / 8 h / 80 °C
6: N-Bromosuccinimide / N,N-dimethyl-formamide / 4 h / 20 °C
7: N-chloro-succinimide / N,N-dimethyl-formamide / 10 h / 20 °C
8: potassium carbonate / N,N-dimethyl-formamide / 3 h / 70 °C
9: sodium hydroxide / water; tetrahydrofuran / 10 h / 45 °C
With
ammonium hydroxide; N-chloro-succinimide; N-Bromosuccinimide; iron; potassium carbonate; ammonium chloride; sodium hydroxide;
In
tetrahydrofuran; methanol; 5,5-dimethyl-1,3-cyclohexadiene; ethanol; water; N,N-dimethyl-formamide;
|
![methyl 5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-1-methyl-1H-benzimidazole-6-carboxylate](/upload/2025/5/0c52efc4-ba65-41c9-91cb-e16e921ca5e0.png)
methyl 5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-1-methyl-1H-benzimidazole-6-carboxylate

![5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-1-methyl-1H-benzimidazole-6-carboxylic acid](/upload/2025/5/6ac43359-42b7-48b4-a206-37032d7b5a53.png)
5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-1-methyl-1H-benzimidazole-6-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
lithium hydroxide;
In
tetrahydrofuran;
at 40 ℃;
for 2h;
Temperature;
|
94.7% |
|
With
lithium hydroxide;
In
tetrahydrofuran;
at 40 ℃;
for 2h;
Temperature;
|
94.7% |
|
With
water; sodium hydroxide;
In
tetrahydrofuran;
at 25 - 30 ℃;
for 12.1667h;
|
88.5% |
|
methyl 5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-1-methyl-1H-benzimidazole-6-carboxylate;
With
sodium hydroxide; water;
In
tetrahydrofuran;
for 2h;
With
hydrogenchloride;
pH=2;
|
79% |
|
methyl 5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-1-methyl-1H-benzimidazole-6-carboxylate;
With
sodium hydroxide; water;
In
tetrahydrofuran;
for 2h;
With
hydrogenchloride;
In
tetrahydrofuran; water;
pH=2;
|
79% |
|
methyl 5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-1-methyl-1H-benzimidazole-6-carboxylate;
With
sodium hydroxide;
In
tetrahydrofuran; water;
for 2h;
With
hydrogenchloride;
In
tetrahydrofuran; water;
pH=2;
|
79% |
|
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 45 ℃;
for 10h;
|
72% |
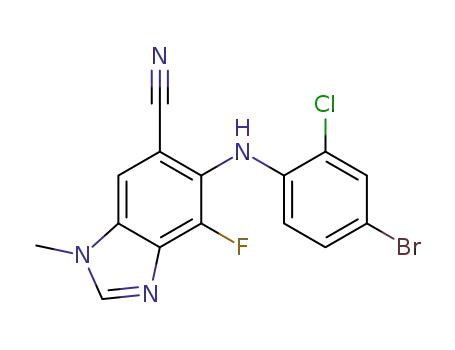
6-(4-bromo-2-chlorophenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carbonitrile
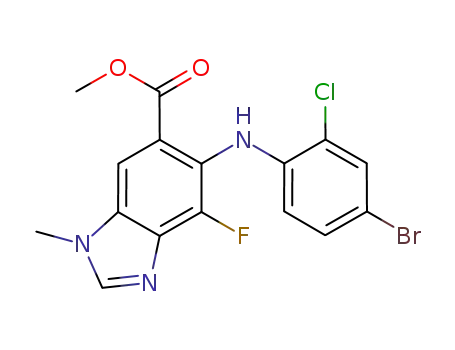
methyl 5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-1-methyl-1H-benzimidazole-6-carboxylate
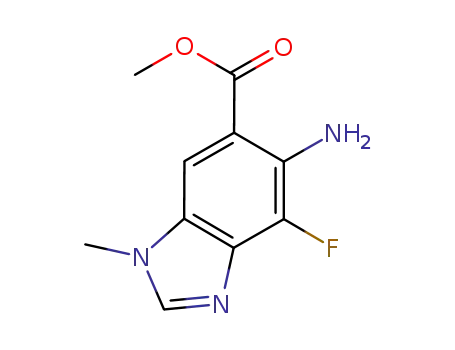
methyl 5-amino-4-fluoro-1-methyl-1H-benzo[d]imidazole-6-carboxylate
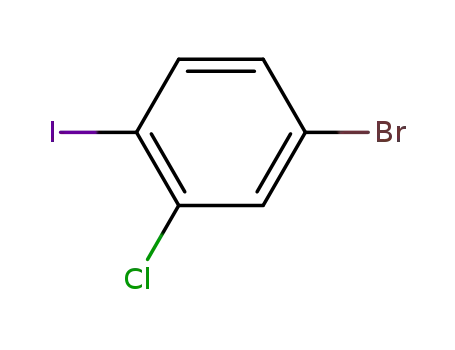
4-bromo-2-chloroiodobenzene
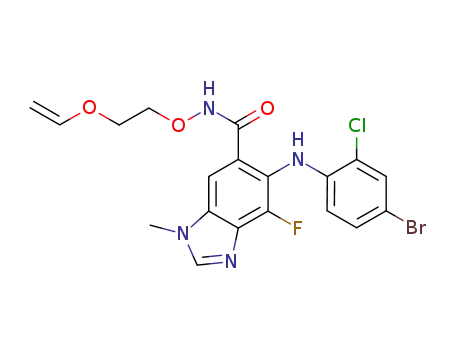
5-[(4-bromo-2-chlorophenyl)amino]-N-[2-(ethenyloxy)ethoxy]-4-fluoro-1-methyl-1H-benzimidazole-6-carboxamide
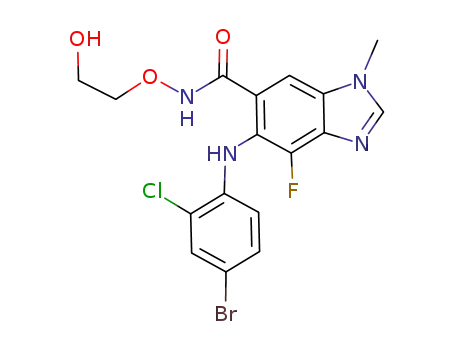
selumetinib