Your Location:Home > Products > Pharmaceutical Intermediates
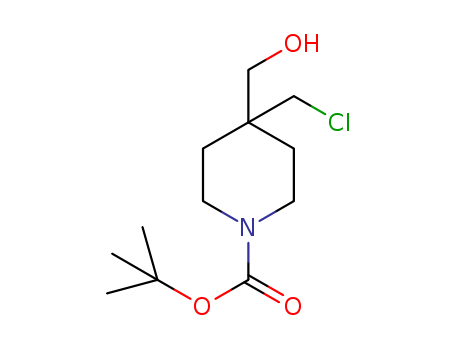

CasNo: 1312131-45-5
MF: C12H22ClNO3
Preclinical and human studies have indic...
The invention discloses a spiro aryl pho...
The optimization for selectivity and cen...
The present invention relates to methods...
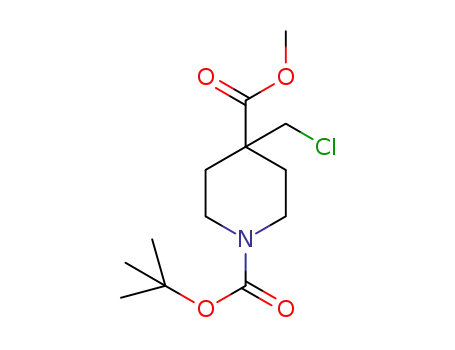
1-tert-butyl 4-methyl 4-(chloromethyl)piperidine-1,4-dicarboxylate

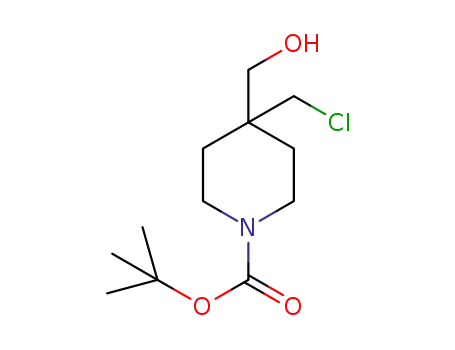
tert-butyl 4-(chloromethyl)-4-(hydroxymethyl)piperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 ℃;
for 0.5h;
|
96% |
|
1-tert-butyl 4-methyl 4-(chloromethyl)piperidine-1,4-dicarboxylate;
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 ℃;
for 0.416667h;
With
water;
In
tetrahydrofuran;
|
93.8% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 ℃;
|
93.8% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 ℃;
for 0.416667h;
|
75.21% |
|
With
lithium aluminium tetrahydride;
|
|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
|
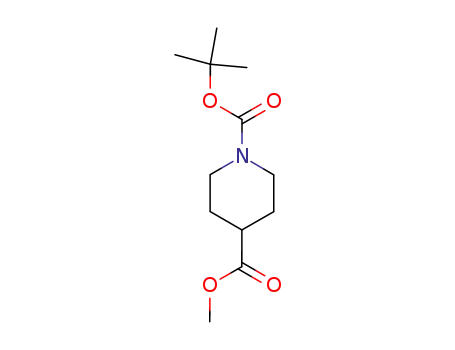
1-tert-Butyl 4-methyl piperidine-1,4-dicarboxylate


tert-butyl 4-(chloromethyl)-4-(hydroxymethyl)piperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: lithium diisopropyl amide
2: lithium aluminium tetrahydride
With
lithium aluminium tetrahydride; lithium diisopropyl amide;
|
|
|
Multi-step reaction with 2 steps
1.1: diisopropylamine; n-butyllithium / tetrahydrofuran; hexanes / 1 h / 0 °C
1.2: 1 h
2.1: lithium aluminium tetrahydride / tetrahydrofuran / 0.42 h / 0 °C
With
lithium aluminium tetrahydride; n-butyllithium; diisopropylamine;
In
tetrahydrofuran; hexanes;
|
|
|
Multi-step reaction with 2 steps
1: lithium diisopropyl amide / tetrahydrofuran
2: lithium aluminium tetrahydride / tetrahydrofuran / 0.5 h / 0 °C
With
lithium aluminium tetrahydride; lithium diisopropyl amide;
In
tetrahydrofuran;
|
|
|
Multi-step reaction with 2 steps
1.1: lithium diisopropyl amide / tetrahydrofuran / 2 h / -78 °C / Inert atmosphere
1.2: 12 h / 20 °C
2.1: lithium aluminium tetrahydride / tetrahydrofuran / 0.42 h / 0 °C
With
lithium aluminium tetrahydride; lithium diisopropyl amide;
In
tetrahydrofuran;
|
|
|
Multi-step reaction with 2 steps
1.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C
1.2: 3 h / 0 °C
2.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C
With
lithium aluminium tetrahydride; lithium diisopropyl amide;
In
tetrahydrofuran;
|

1-tert-butyl 4-methyl 4-(chloromethyl)piperidine-1,4-dicarboxylate

1-tert-Butyl 4-methyl piperidine-1,4-dicarboxylate
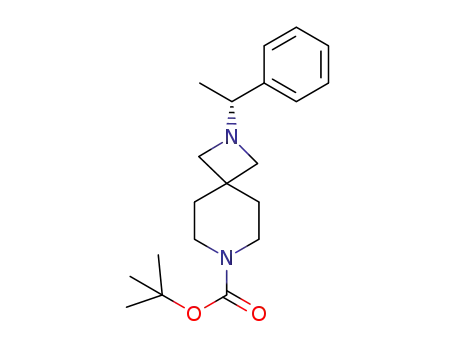
C20H30N2O2
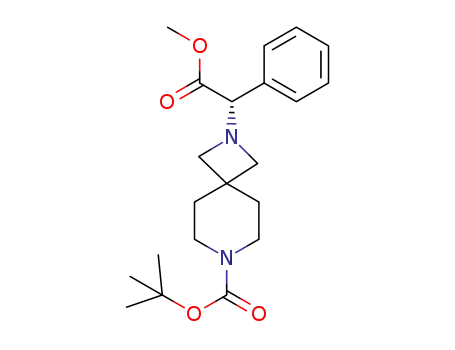
C21H30N2O4
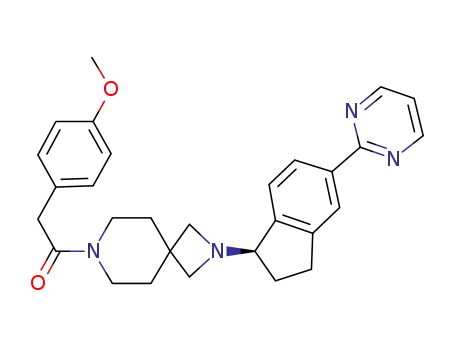
2-(4-methoxyphenyl)-1-{2-[(1R)-5-(pyrimidin-2-yl)-2,3-dihydro-1H-inden-1-yl]-2,7-diazaspiro[3.5]non-7-yl}ethanone
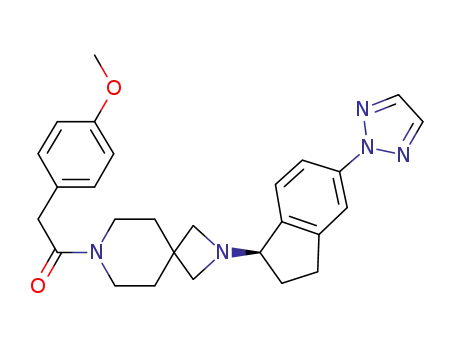
7-[(4-methoxyphenyl)acetyl]-2-[(1R)-5-(2H-1,2,3-triazol-2-yl)-2,3-dihydro-1H-inden-1-yl]-2,7-diazaspiro[3.5]nonane