Your Location:Home > Products > Pharmaceutical Intermediates
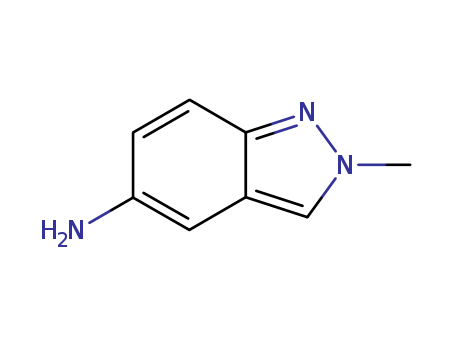

CasNo: 60518-59-4
MF: C8H9 N3
|
General Description |
2-Methyl-2H-indazol-5-amine is a chemical compound with the molecular formula C8H9N3. It is a derivative of indazole, a heterocyclic compound containing a benzene ring fused to a five-membered nitrogen-containing ring. 2-Methyl-2H-indazol-5-amine has potential application in pharmaceutical and medicinal chemistry, possibly in the development of new drugs or as a precursor in organic synthesis. Its specific properties and potential uses would depend on further research and development in the field of chemistry and pharmacology. |
InChI:InChI=1/C8H9N3/c1-11-5-6-4-7(9)2-3-8(6)10-11/h2-5H,9H2,1H3
The present invention relates generally ...
The development of straightforward acces...
Described herein are compounds of Formul...
The present invention provides 2,4-pyrim...
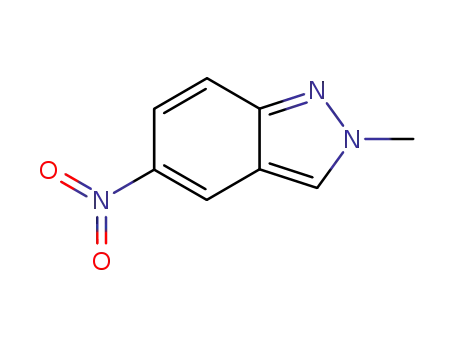
2-methyl-5-nitro-2H-indazole

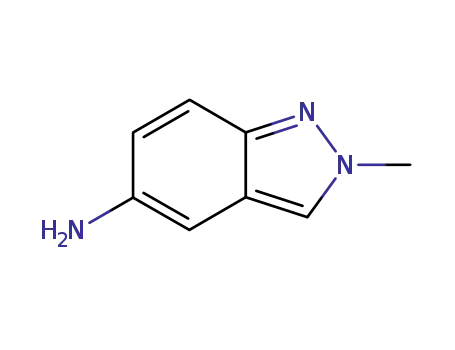
5-amino-2-methyl-2H-indazole
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium on activated charcoal;
In
methanol; dichloromethane;
at 50 ℃;
under 2250.23 Torr;
|
98% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol; dichloromethane;
at 50 ℃;
for 3h;
under 2585.81 Torr;
|
85% |
|
With
hydrogen;
palladium;
In
ethanol;
under 836 Torr;
|
80% |
|
With
3% Pd/C; hydrogen;
In
methanol; dichloromethane;
at 50 ℃;
for 6h;
under 18751.9 Torr;
|
|
|
With
iron; ammonium chloride;
In
ethanol; water;
for 2h;
Reflux;
|
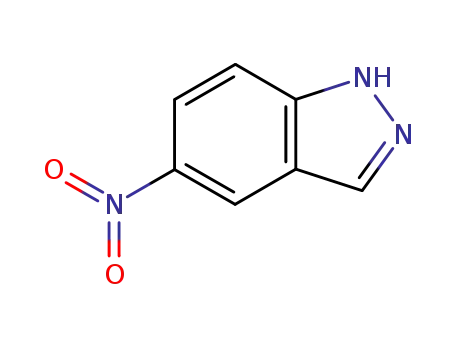
5-nitroindazole


5-amino-2-methyl-2H-indazole
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 44 percent / KOH; water / 0.5 h / 75 °C
2: 20 percent / conc. HCl; SnCl2*H2O / methanol / Heating
With
hydrogenchloride; potassium hydroxide; water; tin(ll) chloride;
In
methanol;
|
|
|
Multi-step reaction with 2 steps
1: 44 percent / KOH; water / 0.5 h / 75 °C
2: 98 percent / H2 / Pd/C / methanol; CH2Cl2 / 50 °C / 2250.23 Torr
With
potassium hydroxide; water; hydrogen;
palladium on activated charcoal;
In
methanol; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: 43 percent / potassium hydroxide / H2O / 45 °C
2: 80 percent / H2, buffer (pH = 7) / 10percent Pd / ethanol / 836 Torr
With
potassium hydroxide; hydrogen;
palladium;
In
ethanol; water;
|
|
|
Multi-step reaction with 2 steps
1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 0 - 20 °C
2: ammonium chloride; iron / water; ethanol / 2 h / Reflux
With
iron; sodium hydride; ammonium chloride;
In
ethanol; water; N,N-dimethyl-formamide;
|

2-methyl-5-nitro-2H-indazole

5-nitroindazole
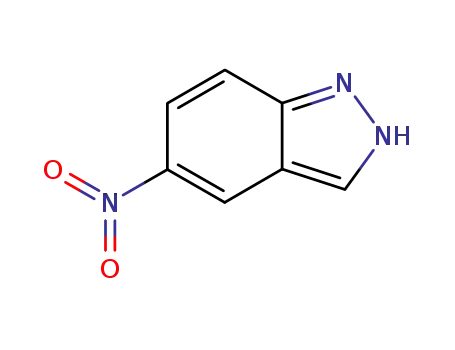
5-nitro-2H-indazole